Page Synopsis: This page is a diifcult read, easier to just skim the titles and conclusions
Troxerutin cerebroprotein hydrolysate injection ameliorates neurovascular injury induced by traumatic brain injury
https://pubmed.ncbi.nlm.nih.gov/29883225
TCH resulted in alleviation of neurological deficits, reduction of infarct volume, improvement of regional cerebral blood flow (rCBF), amelioration of neuronal death, astrocyte proliferation, endothelial cell loss, and BBB dysintegrity. These effects of TCH treatment against TBI were through endothelial nitric oxide synthase (eNOS) coupling/decoupling status adjustment, which not only increased nitric oxide (NO) level, but also decreased peroxynitrate level expression.
Conclusions: 'All the results indicated that TCH injection has multifaceted protective effects of neurovascular unit (NVU) against TBI via eNOS pathway regulation'
v
Skill Level 5
Relevance:5 Technical Level:5
page 62b PTBICF > ALLOPATHIC MEDICINE > TROXERUTIN FLAVONOID and CEREBROPROTEIN HYDROLYSATE (TCH
page 62
page 63
Another topic your doctor probably hasn't mentioned that may be supremely helpful
A retrospective chart analysis of 34 cases with the use of oral cerebroprotein hydrolysate in dementia in a tertiary general hospital https://www.jgmh.org/article.asp?issn=2348-9995;year=2021;volume=8;issue=1;spage=30;epage=33;aulast=Karia
Cerebroprotein is the first drug that is neurotrophic in nature in the laboratory and promotes neuronal survival and growth.[15] It is peptide in nature just like many endogenous neurotrophic factors. Studies have documented neuroprotective and neurotrophic action with injectable medication but studies with the oral preparation are scarce.[16] This drug is the only medication acting at a neuronal level unlike others that act at neurotransmission and neurotransmitter level in dementia.[17] In the cases mentioned above, we continued the medications that patients were on Cerebroprotein and especially in those where improvement was noted. We were surprised to see improvement in areas of bladder and bowel control, aggressive behavior, pathological laughter, verbal disinhibition, and especially as these areas had shown no improvement so far in these cases. It was difficult to comment whether this improvement was due to oral cerebroprotein or due to other drugs or spontaneous remission. It is worthwhile noting that the improvement was seen however only 7–10 days after oral cerebroprotein was started. We admit that changes in cognition and memory seen with oral cerebroprotein was mild to moderate and yielded scores within the dementia range itself, but still a positive change in scores were noted. No major side effects were seen in our cases. Cerebroprotein is currently available both as an injectable and as an oral preparation. It has been mentioned that the injectable drug works faster than the oral one but patient preference is for an oral dosage. It is worthwhile that the oral drug be tried as an add-on with other current available treatments in dementia. Further longitudinal studies with the oral preparation and comparing it with the injectable preparation in varied cohorts of dementia are warranted. The study had limitations as it was restricted to 34 patients in a retrospective chart analysis. The study was an open one and no blinding was done. There was no control group in the study
![]()
Transcriptome Analysis Reveals the Effects of Troxerutin and Cerebroprotein Hydrolysate Injection on Injured Spinal Cords
https://www.hindawi.com/journals/ecam/2020/3561235
TCH injection with the right dose is effective for the recovery of locomotion function and repairing of the damaged tissue in the spinal cord; TCH injection is also discovered to have a role in the regulation of 443 differentially expressed genes (DEGs) and 27 differentially expressed LncRNAs (DELs) that are identified to have multiple functions, including locomotion, blood vessel morphogenesis, thiamine metabolism, Hippo signaling pathway, and axon guidance, by applying the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) analysis. In addition, it is revealed that, after SCI, the highly expressed LncRNA AABR07071383.1 in the post-SCI cis/trans-regulates the expression of mRNA Acpp mRNA that encodes a key enzyme involved in the metabolic process of thiamine in the abirritation of the dorsal root ganglion (DRG), which implies that TCH injection may be more effective when administered with benfotiamine
Troxerutin is a derivative of bioflavonoid rutin that is widely distributed in fruits, vegetables, and grains [17]. There is mounting clinical evidence to show that troxerutin possesses pharmacological effects in the treatment of multiple diseases. It has been revealed that troxerutin improves symptoms of insulin resistance and hyperlipidemia in diabetes via antioxidant activity. Previous studies also showed that troxerutin protects against 2′,2′,4,4′-tetrabromo diphenyl ether (BDE-47) mediated inflammation damage to ameliorate the antioxidant level after traumatic injuries of various pathological conditions such as an acute kidney injury and TBI [11, 18, 19]. Troxerutin has been proven to be beneficial in the treatment of neuropsychological diseases; oral administration of troxerutin could reverse synaptic failure and cognitive disorder in the mice model of Alzheimer’s disease [17, 20]. In addition, troxerutin has been shown to be effective in the treatment of hemorrhoidal diseases where it protects the endothelial cells and improves local microcirculation [10, 21]. In a previous study, 15 DEGs were identified by transcriptomic analysis of the blood cells of rats treated with troxerutin [8]; this demonstrated that troxerutin mainly possesses functions of T-cell-mediated cytotoxicity, telencephalon development, cell membrane development, endoplasmic reticulum exports, β2-microglobule binding, and transporter associated with antigen presentation (TAP)-binding. Cerebroprotein hydrolysate, also known as Cerebrolysin [22], is suggested to play a role as the precursor to a neurotransmitter; it also directly functions as a neurotransmitter and is used to improve the recovery of neurological function [9, 22, 23]. Due to its ability to penetrate the blood-brain barrier easily, it is widely used in clinical treatment for disorders of the central and peripheral nervous system such as stroke and nerve injuries either separately or in combination with other methods [12, 24]. TCH has been extensively used in clinics in China [10] and has shown to inhibit the process of oxidative stress and promote angiogenesis in cerebral ischemia. It also has an effect on the recovery of TBI by protecting neurovascular units from reoxygenation-induced injury [10].
The goal of the recovery process in SCI is to have a partial or full restoration of motor and sensation nerve conduction [25]; this is very difficult to achieve because there are several factors that accelerate neuron necrosis and impede the functional regeneration of spinal cord neuronal axons [26]. Neurons in the post-SCI microenvironment lack essential growth facilitators [27], such as the signaling pathways involved in axon regeneration, synapse remodeling, and circuit reorganization. Studies have shown that the activation of axon regeneration signaling pathways like WNT and Hippo and the upregulation of attractive and repulsive cues related to molecules in the local environment such as netrins and semaphorins could quicken the recovery sensory deficit and dyskinesia [28–32]. From the results of this study, it can be seen that when the concentration of TCH exceeds 6 ml/kg, the efficacy of TCH decreases and the drug concentration dependence disappears, which indicates that there is a concentration dependence of TCH in a certain concentration range. Therefore, the dose of 6 ml/kg is the balance between the maximum efficacy and the lowest side effects of TCH. In this study, we discovered for the first time that the recommended therapeutic dose of TCH after SCI in rats is 6 ml/kg. Within 2 weeks after administration of this dose of TCH to SCI rats, it was found that further injury could be prevented by reducing neuronal apoptosis and promoting the recovery of neurological function. The GO annotation analysis on the results of RNA-seq suggested that 192 upregulated genes were associated with developmental processes, 162 upregulated genes were associated with signaling, 79 upregulated genes are associated with locomotion, 10 upregulated genes were associated with synapse, and 3 upregulated genes were associated with antioxidant activity.
The KEGG enrichment analysis showed that the Rno04390 Hippo signaling pathway and Rno04360 axon guidance were highly enriched. 4 DEGs were considered to be involved in the TCH effect; troxerutin may have downregulated the expression of caspase-6 (Casp6), a downstream enzyme in the activation cascade. The reactivation of Casp6 was found to have a strong correlation to cell death and specific roles in the CNS, especially in the case of neurodegenerative disorders such as axonal degeneration and Alzheimer’s disease [33]. It has also been observed that the inhibition or knockdown of Casp6 would delay the course of axon damage and neurofilament dissolution [34]. Bone morphogenetic protein 7 (Bmp7) induces ectopic bone formation by potently inhibiting TNF-α-induced oligodendrocyte apoptosis [35]. It is also highly expressed in a number of glial cells and motor neurons after spinal cord injury, which indicates that the upregulation of Bmp7 can promote nerve repair; previous studies have confirmed that local injection of Bmp7 can promote both neuronal regeneration after spinal cord injury and the recovery of motor function in rats [36].
Netrin-1 (Ntn1) has a strong chemotropic function for axonal guidance, cell migration, morphogenesis, and angiogenesis in CNS [32]. In the peripheral nervous system, Ntn1 receptors are expressed in Schwann cells, the cell bodies of sensory neurons, and the axons of both motor and sensory neurons that cause dissociation of UNC5C in microtubules and lead to axon repulsion [31, 32]; Ntn1 also promotes the projection of axon in DRG towards the spinal cord [37]. It could also prevent the initiation of apoptosis; studies show that Ntn1 is downregulated after an axon injury and that the application of exogenous netrin-1 after TBI normalizes spine density and presynaptic excitability of the injured neurons [32]. However, its expression is upregulated after a peripheral nerve transection injury [31].
The other challenge in the recovery of SCI is that the constriction of blood vessels by the action of pericytes leads to acute ischemia [38]. In the long term, pericyte 5-HT1 and α2 adrenergic receptors become activated, which would also constrict capillaries, thereby causing a chronic state of ischemia and hypoxia locally for several months as seen in a rat SCI model [39, 40]. It has also been proven that increased blood vascular density and restored blood supply could promote neuronal survival, axonal regeneration, and functional recovery. The results of the present study showed that TCH stabilized the injured segment by reducing posttraumatic spinal cord edema. The GO annotation in RNA-seq results suggested that 129 upregulated genes were associated with the extracellular region part, 138 upregulated genes are associated with negative regulation of biological processes, 79 upregulated genes were associated with locomotion, and 81 upregulated genes were associated with cell proliferation. The GO enrichment analysis showed that GO: 0048514 (blood vessel morphogenesis) and GO: 0009888 (tissue development) were highly enriched. Three DEGs were considered to be involved in the TCH effect, and Cyr61 is a secreted protein that interacted with heparan sulfate proteoglycan and integrins. The expression of Cyr61 was induced by growth factor and its high expression played a role in cell proliferation, angiogenesis, and extracellular matrix formation.
Various mechanical stresses induce Cyr61 expression in cartilage/bone tissues and in periodontal ligaments. Cyr61 also engages in a distinct intracellular signaling cascade in microglia/macrophages and promotes M1 macrophage recruitment in the compressed spinal cord [41]. Our present data indicated that Cyr61 was significantly upregulated in the chronically, severely compressed spinal cord and that it colocalized extensively with reactive astrocytes at sites of inflammation. SMOC-2 is highly expressed during wound healing; it could stimulate endothelial cell proliferation and angiogenic activity, thus serving as a target for angiogenesis in myocardial ischemia [42]. Foxc2 plays a role in the development of mesenchymal tissues by acting as a crucial modulator during both angiogenesis and lymphangion genesis. It has been revealed that the expression of Foxc2 was elevated by hypoxia in the postischemia/reperfusion injury in the kidneys; it facilitates vascular development and modulates a variety of angiogenic factors, including Mmp2, Pdgfb, Vegf, Dll4, Notch1, and Hey2 [43]. It can also regulate the expression of osteopontin, which is a determinant of PI-induced tumor angiogenesis. Foxc2 is also found to activate the ERK or PI3K signaling pathway in the bone marrow mesenchymal stem cell-induced angiogenesis [44].
In the present study, a total of 21 upregulated and 7 downregulated DELs were identified in the TCH treated group after SCI as compared to the control group. The regulation analysis of LncRNA-mRNA revealed that mRNA of Ksr1, Ddi2, Wnt4, Sik1, Acpp, and Nfatc4 were potentially trans-regulated by LncRNA AABR07071383.1 while Prg4, Cdh11, Fbn2, Hmcn1, and Acpp were cis-regulated by LncRNA AABR07021357.1, AABR07042668.1, AABR07072602.1, AABR07021357.1, and AABR07071383.1, respectively.
We focused our attention on the trans/cis-regulation pair of LncRNA AABR07071383.1 and mRNA Acpp. Benfotiamine (S-benzoylthiamine O-monophosphate, BT) was observed to have antinociceptive effects in animals and Acpp (Prostatic acid phosphatase) could dephosphorylate BT in the DRG neurons of the spinal cord. The dephosphorylated product S-benzoylthiamine (S-BT) decomposes to O-benzoylthiamine (O-BT) and finally to thiamine, thus showing antinociceptive effects in animals and humans [45–47]. Our results suggested that TCH may have a synergistic effect with benfotiamine as TCH improves the expression of LncRNA AABR07071383.1, which upregulates the expression of Acpp and accelerates the metabolic process of benfotiamine, thus having antinociceptive effects in the post-SCI condition (Figure 12).
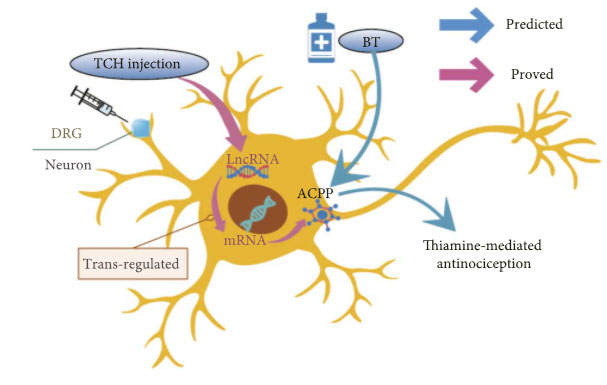
Schematic diagram of the hypothetic molecular mechanism of TCH injection having a synergistic effect to benfotiamine analgesic action after SCI.
However, a limitation of this study was that the sample size for RNA-seq was small (n = 2 for each group); this was due to the requirement of high-quality data for bioinformatic analysis, which was hard to obtain. However, it did not impact the analysis for DEGs and DELs or the function analysis as it has been shown that the false positive rate of transcriptome analysis does not decrease substantially as the sample size increases in the condition of n ≥ 2, with the proper depth of sequencing [48], which was qualified in the study.
In conclusion, the findings of this study suggested that TCH injection is effective for the recovery of neural function and local angiogenesis in rats after SCI; the transcriptome analysis showed that TCH regulated the expression of genes that mediate multiple functions such as axon regeneration and blood vessel morphogenesis in the spinal cord tissue. The construction of the LncRNA-mRNA networks showed that TCH mediated the expression of Acpp by upregulating LncRNA, which implies the potential synergistic effect of abirritation that TCH and BT may have for the treatment of SCI; this provides a theoretical basis for the use of TCH injection in clinical practice and investigation
Troxerutin (Vitamin P4) for the Treatment of Chronic Diseases https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7903491
Troxerutin (TRX), a semi-synthetic bioflavonoid derived from rutin, has been reported to exert several pharmacological effects including antioxidant, anti-inflammatory, antihyperlipidemic, and nephroprotective. However, the related molecular details and its mechanisms remain poorly understood. In the present review, we presented evidences from the diversity in vitro and in vivo studies on the therapeutic potential of TRX against neurodegenerative, diabetes, cancer and cardiovascular diseases with the purpose to find molecular pathways related to the treatment efficacy.
TRX has a beneficial role in many diseases through multiple mechanisms including, increasing antioxidant enzymes and reducing oxidative damage, decreasing in proapoptotic proteins (APAF-1, BAX, caspases-9 and-3) and increasing the antiapoptotic BCL-2, increasing the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and downregulating the nuclear factor κB (NFκ). TRX also reduces acetylcholinesterase activity and upregulates phosphoinositide 3-kinase/Akt signaling pathway in Alzheimer’s disease models. Natural products such as TRX may develop numerous and intracellular pathways at several steps in the treatment of many diseases. Molecular mechanisms of action are revealing novel, possible combinational beneficial approaches to treat multiple pathological conditions
![]()
Antifatigue effects of troxerutin on exercise endurance capacity, oxidative stress and matrix metalloproteinase-9 levels
https://pubmed.ncbi.nlm.nih.gov/28214375
TRX significantly increased glucose level (P < 0.05) and reduced creatine kinase activity (P < 0.001) compared to the vehicle and exercise groups. TRX300 significantly reduced alkaline phosphatase and lactate dehydrogenase activities (P < 0.05) and blood urea nitrogen (P < 0.05) and MMP-9 levels (P < 0.05) compared to the vehicle and exercise groups. Additionally, TRX300 and TRX150 significantly increased superoxide dismutase activity compared to the vehicle group (P < 0.05). Our results provide experimental evidence in supporting clinical use of TRX as an effective agent against fatigue
![]()
The Cerebroprotein Hydrolysate-I Plays a Neuroprotective Effect on Cerebral Ischemic Stroke by Inhibiting MEK/ERK1/2 Signaling Pathway https://www.proquest.com/docview/2552746619
Acute ischemic stroke is a common type of stroke.1,2 Ultra-early thrombolytic therapy is currently recognized as the main operative treatment, but the treatment time window of this therapy is only 3–6 hours, and there are many complications after thrombolytic therapy,2,3 which limits its clinical application. Neuronal damage in the ischemic penumbra or peri-infarct area is transient and reversible in the first few hours after ischemia.2
A series of studies have shown that the application of neuroprotective drugs as adjuvant therapy of thrombolytic therapy can contribute to improving the neurological function of patients with ischemic stroke.4–6 Cerebroprotein hydrolysate-I (CH-I), a mixture, was prepared from denatured proteins with a total nitrogen content of more than 120 mg/g.7 It contained 16 kinds of amino acids and 25–35% small molecule polypeptides (<10,000 Da).7 It also contained many nerve growth factors, which have similar pharmacodynamic characteristics with endogenous neurotrophic factors, and have neurotrophic and neuroprotective effects.8–11 It has been found that CH-I and cerebrolysin (CBL) have similar effects to brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), and have neuroprotective effects such as reducing the inflammatory response, apoptosis, and encephaledema.8,9,11
BDNF is a protein synthesized in the brain, which is widespread in the central nervous system and can promote the recovery of function after stroke.12 It has been noted that the expression of BDNF is decreased after stroke, and the neurobehavioral function of cerebral ischemia rats can be improved by up-regulating BDNF expression.13 Mature BDNF dimer binds to tyrosine kinase receptor B (TrkB) to induce autophosphorylation of tyrosine residues and then regulate intracellular signaling pathways: Mitogen-activated protein kinase/Extracellular signal-regulated protein kinase (MAPK/ERK1/2), which leads to the phosphorylation and activation of downstream cAMP response element binding protein (CREB), and further mediates gene transcription of neurons.14,15
Ischemia/reperfusion (I/R) is a complex process, which refers to the interruption of blood flow to tissues and then recovery.16 Inflammation, oxidative stress, and apoptosis were important pathophysiological pathways after I/R injury.16–18 The MAPK signaling pathway is a major signaling pathway in the process of cerebral ischemia reperfusion injury (CIRI), and MAPK kinase (MEK)/ERK is an important part of the MAPK signaling pathway.19 CIRI can activate the MEK/ERK1/2 signaling pathway, which can reduce brain nerve cell death and improve prognosis by inhibiting the MEK/ERK1/2 signaling pathway.19,20 This study sought to investigate whether CH-I could play a neuroprotective effect against CIRI by intervening the MEK/ERK1/2 signaling pathway and downstream CREB pathway
![]()
Cerebroprotein hydrolysate allergy in patients with traumatic brain injury https://asja.springeropen.com/articles/10.1186/s42077-021-00180-4#Sec3
The neurotrophic factors are small proteins that exert trophic actions on neuronal cells. They are nerve growth factor, glial cell-derived neurotrophic factor, brain-derived neurotrophic factor, neurotrophin 3, growth-associated protein, and ciliary neurotrophic factor. Cerebroprotein enhances the neuronal survival by enhancing the effect through calfin. It provides neuromodulatory action and repair of neurons and has neuroimmunotrophic action (Sharma et al. 2010). It decreases the beta amyloid deposition used in Alzheimer’s disease (Plosker and Gauthier 2009); it modulates the neuronal plasticity and is used in traumatic brain injury (Wong et al. 2005) and vascular injury. It helps in the differentiation of neurons and protects against ischemia and neurotoxic injury. Common side effects include headache, agitation, fever, chills, flu-like syndrome, hallucination, and confusion. There is drug interaction with monoamine oxidase inhibitors. The neurotropic activity in plasma is detected after 24 h after a single injection
please wait for document to load, use sidebar to scroll down page
click here to read the entire document as a continuous file (or if document takes too long to load)
![]()
Cerebroprotein Hydrolysate: Innovation in Neurodegenerative Disorders
treatment of neurodegenerative disorders is to develop drugs to stimulate nerve repair itself.
Several drugs like Edaravone, Citicoline and Piracetam have been developed based on neurotrophic factors. Neurotrophic factors are small proteins that exert survival-promoting and trophic actions on neuronal cells.
These neurotrophic factors are NGF (Nerve growth factor), BDNF (Brain-derived neurotrophic factor), NT-3 (Neurotrophin-3), GDNF (Glial cell-derived neurotrophic factor), GAP-43 (growth associated protein 43) and CNFT (Cilliary neurotrophic factor). Glial cells continue to undergo cell division in adulthood and their ability to proliferate is particularly noticeable after brain injury (e.g stroke).
This is not the case with neurons; they cannot divide themselves but they undergo a lot of activity after injury. Interestingly, studies demonstrate that neurons in the adult brain have an unappreciated capacity for remodeling away from the actual injury, and that these neurons are attempting to rewire the brain following an injury.
Cerebroprotein hydrolysate is the latest weapon in the physician’s armamentarium. It is a neurotrophic drug. It consists of short biological peptides which act like endogenous neurotrophic factors
Pharmacokinetics
It is given in a dose of 60 -180 mg once daily for 10-20 days. It should be slowly infused in 250 ml saline in 60-120 minutes. Maintenance doses (30 mg) can be given by i.m route. It should not be mixed with amino acid solutions in the infusion bottle. Doses of antidepressants should be reduced if used with Cerebroprotein hydrolysate.
Adverse effects and Contraindications
Studies have revealed that most of the side effects are minor. Most common side effects include headache, nausea, vertigo, increased sweating, agitation, fever, hallucinations, confusion, and flu like syndrome. Contraindications include hypersensitivity, epilepsy and severe renal impairment. Safety has not been established in pregnancy and lactation.
Indications
Acute ischemic stroke
Traumatic brain injury
Vascular dementia
Alzheimer’s disease
Mechanism of action and pharmacological effects
It acts by multiple mechanisms viz.—
- Regulation and improvement of the neuronal metabolism.
- Modulation of the synaptic plasticity.
- Neuronal differentiation and protection against ischemic and neurotoxin lesion
Cerebroprotein hydrolysate reduces excitotoxic damage, blocks over activation of calcium dependent proteases, and scavenges free oxygen radicals. Cerebroprotein hydrolysate has been shown to counteract the negative effect of
the elevated EGF-2 on neurogenesis and neuromodulation.
Cerebroprotein hydrolysate -augmented proliferation, differentiation, and migration of adult sub ventricular zone (SVZ) neural progenitor cells results in increased number of neural progenitor cells and neuroblasts to contribute to neurogenesis. This may be the mechanism for beneficial effect in acute ischemic stroke and traumatic brain injury. Enhancement of neuronal survival is produced through effect on calpain. The hyper activation of calpain is implicated in a number of neurodegenerative disorders. Calpain is inhibited by Cerebroprotein hydrolysate
Neuromodulatory effect is produced by increasing glucose transporter1 (GLUT-1) expression which is responsible for more than 90% of glucose transport to brain.
Neuronal plasticity is produced by reduction of amyloid beta accumulation, increased MAP 2 and Synaptophysin synthesis. Neuro-immunotrophic activity is produced by inhibition of microglial activation and expression of IL-1 beta. This results in reduction of inflammation. Other neurotrophic drugs and nootropics are not having so much broad spectrum of different actions possessed by Cerebroprotein hydrolysate. The patients of neurodegenerative disorders now can be managed in a better way with the advent of Cerebroprotein hydrolysate
ReferencesLarry BG, Robert A, Kyra B, Curt DF, Philip BG, George H et al. Primary Prevention of Ischemic Stroke. A Statement for Healthcare Professionals from the Stroke Council of the American Heart Association. Stroke 2001;32:280-99. 2. Injury Prevention & Control: Traumatic Brain Injury. Available from:http:// www.cdc.gov/traumaticbraininjury/. [last accessed on 30-nov-2011]. 3. Aziz-Sultan A, Baimeedi P, Benzel EC, Berger MS, Brandner S, Butowski N et al. Introduction to traumatic brain injury. In Samandouras G, editor. Traumatic brain injury: The Neurosurgeon's Handbook. Ist ed. Oxford: Oxford University Press; 2010.p.207-8. 4. Sanjeev V Thomas. Addressing problems of dementia in India. Ann Indian Acad Neurol 2011;14:147. 5. Introduction to neurogenesis. Available fromhttp://www.neurogenesis .iord.org /.[Last accessed on 15th Oct. 2011]. 6. Available from: http://www.ceregene. com / neurotrophic.asp. [Last accessed on 30 Oct. 2011]. 7. Barrett KE, Barman SM, Boitano S, Brooks HL. Excitable Tissue: Nerve. In Ganong WF, editor. Physiology of nerve and muscle cells: Review of Medical Physiology. 23rd ed. Lange: Mc Graw Hill; 2010.p.80. 8. Gopalan T. Neurons: Key To Healing Of Brain Injuries Identified. August 24, 2010. Research News [online] Available from: http://www.medindia.net /news /Neurons-Key-To- Healing-Of-Brain-Injuries-Identified-73058-2.htm. [Last accessed on Nov 28 2011]. 9. Long J, Wei GZ, Xu ZB, Feng YB. Injection of cerebroprotein hydrolysate vs Cerebrolysin in treating cerebrovascular diseases. Available from: http://en. cnki.com.cn/Article_en / CJFDTOTALSui CM, Kam WC, Jia-Hong L, Durairajan SSK, Liang-Feng L, Min L. Systematic Review on the Efficacy and Safety of Herbal Medicines for Vascular Dementia. Available from: http:// www. ncbi.nlm.nih.gov/pmc/articles/PMC3250997. [Last accessed on March 07 2012]. 11. Honghui C, Tung YC, Li B, Iqbal K, Iqbal IG Trophic factors counteract elevated EGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging 2007 Aug;28(8):1148-62. 12.Ruben JB, Dafang Wu, Manfred W. In Vivo upregulation of the blood–brain barrier GLUT1 glucose transporter by brain-derived peptides
![]()
Cerebroprotein hydrolysate in traumatic brain injury
https://www.researchgate.net/publication/276173777_Cerebroprotein_hydrolysate_in_traumatic_brain_injury
CH may prove to be beneficial in such patients. A complex study of cognitive and emotional status, levels of serum serotonin and brain-derived neurotrophic factor performed in 72 patients with acute TBI, with a special focus on middle brain injuries, treated with cerebrolysin found that it promotes activation of neurotrophic pro-cesses and improves outcomes of closed craniocerebral injury [9]. A double-blind, placebo-controlled, randomized study showed that cerebrolysin improves the cognitive function of patients with mild TBI at 3rdmonth after injury, especially for long-term memory and drawing function tested on MMSE and Cognitive Abilities Screening Instrument (CASI) scores
v
page 62b PTBICF > ALLOPATHIC MEDICINE > TROXERUTIN FLAVONOID and CEREBROPROTEIN HYDROLYSATE (TCH
page 62
page 63












