Page Synopsis: full blood transfusion therapy (BTT) has not yet been trialled for CFS, though BTT has proven successful for 'other' autoimmune illnesses (though it remains unclear to what degree CFS ought to be considered entirely an autoimmune illness). As BTT has been used successfully in covid patients (where long covid overlaps with CFS), it seems a logical next step (something to watch for).
IVIG (a transfusion product, not the full transfusion) has long been used for CFS with mixed results (to read about IVIG view full report at
https://bra.in/4qeDrg and https://bra.in/9pW6Eo
BTT may also address some of the Circulatory issues found with CFS patients click here to scroll to bottom of page where issues are listed
Skill Level 4
Relevance:2 Technical Level:5
The Effect of Red Blood Cell Transfusion on Fatigue in Hospitalized Patients with Anemia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6191327
Transfusion interacted with nadir Hb was associated with reduced fatigue post-discharge for patients with higher baseline fatigue (20% most fatigued: β=12, p=0.02; 10% most fatigued: β=17, p=0.02). Patients <50 years old with high baseline fatigue had large reductions in fatigue from transfusion (20%: β=23, p=0.02; 10%: β=29, p=0.03).
Conclusions:
Transfusion during hospitalization is associated with reduced fatigue 30 days post-discharge in patients with higher levels of baseline fatigue
![]()
Platelet transfusion therapy and circulating immune complexes
https://pubmed.ncbi.nlm.nih.gov/7445473
30 platelet transfusions were administered to 9 thrombocytopenic patients with acute leukemias. Using the Raji cell radioimmunoassay, the serum concentration for circulating immune complexes (CIC) were determined immediately before and 10--12 h after each transfusion therapy. Elevated serum concentrations for CIC were present in 14 of the pretransfusion samples. After platelet transfusion therapy, a significant (p < 0.02) decrease in the values of CIC occurred. In these 14 transfusion studies the mean percent platelet increment was considerably lower (0.10 > p > 0.05) than the mean for the 16 transfusion studies in which the pretransfusion values for CIC were normal. These results show that CIC probably play an important role in removing transfused platelets from the circulation. Conversely, platelets are able to remove CIC from the circulation
![]()
When the body fights its own red blood cells
https://www.ihtc.org/autoimmune-hemolytic-anemia
red blood cell transfusion support. This corrects the severe anemia which could be life threatening. A blood transfusion does not correct the underlying cause of the AIHA, but it prevents serious complications from severe anemia.
Treatment for AIHA is directed at getting rid of the antibody. This can be done a couple of different ways. There are oral medications that can be given to suppress the body’s ability to make the antibody. This acts to “quiet” the person’s overactive immune system.
Another approach is to use intravenous medications to “trick” the spleen into ignoring the antibody coated red blood cells as they circulate.
Another way to get rid of the antibody is to remove it with an intravenous treatment called pheresis (for-e-sis). This involves washing the blood through a machine that has an “antibody magnet” to attract and destroy the antibodies, then return the normal cells back
Convalescent plasma therapy https://www.mayoclinic.org/tests-procedures/convalescent-plasma-therapy/about/pac-20486440
Convalescent plasma (kon-vuh-LES-unt PLAZ-muh) therapy uses blood from people who've recovered from an illness to help others recover.
The U.S. Food and Drug Administration (FDA) has given emergency authorization for convalescent plasma therapy with high antibody levels to treat COVID-19. It may be used for some hospitalized people ill with COVID-19 who are either early in their illness or who have weakened immune systems.
Blood donated by people who've recovered from COVID-19 has antibodies to the virus that causes it. The donated blood is processed to remove blood cells, leaving behind liquid (plasma) and antibodies. These can be given to people with COVID-19 to boost their ability to fight the virus.
Why it's done
Convalescent plasma therapy may be given to people with COVID-19 who are in the hospital and are early in their illness or have a weakened immune system.
Convalescent plasma therapy may help people recover from COVID-19. It may lessen the severity or shorten the length of the disease.
About
Risks
Blood has been used to treat many other conditions. It's usually very safe. The risk of getting COVID-19 from convalescent plasma hasn't been tested yet. But researchers believe that the risk is low because donors have fully recovered from the infection.
Convalescent plasma therapy has some risks, such as:
Allergic reactions
Lung damage and difficulty breathing
Infections such as HIV and hepatitis B and C
The risk of such infections is low. Donated blood must be tested for safety. Some people may have mild complications or none at all. Other people may have severe or life-threatening complications.
What you can expect
Your doctor may consider convalescent plasma therapy if you're in the hospital with COVID-19 and you are early in your illness or you have a weakened immune system. If you have questions about convalescent plasma therapy, ask your doctor.
Your doctor will order convalescent plasma that is compatible with your blood type from your hospital's local blood supplier.
Before the procedure
Before convalescent plasma therapy, your health care team prepares you for the procedure. A health care team member inserts a sterile single-use needle connected to a tube (intravenous, or IV, line) into a vein in one of your arms.
During the procedure
When the plasma arrives, the sterile plasma bag is attached to the tube and the plasma drips out of the bag and into the tube. It takes about 1 to 2 hours to complete the procedure.
After the procedure
You'll be closely monitored after you receive the convalescent plasma. Your doctor will record your response to the treatment. He or she may also record how long you need to stay in the hospital and if you need other therapies.
Results
It's not yet known if convalescent plasma therapy will be an effective treatment for COVID-19. You might not experience any benefit. However, this therapy might help you recover from the disease.
Data from several clinical trials, studies and a national access program suggest that convalescent plasma with high antibody levels may lessen the severity or shorten the duration of COVID-19 in some people when given early in the disease or in those with weakened immune systems. However, more research is needed to determine if convalescent plasma therapy will be an effective treatment for COVID-19.
![]()
Blood Transfusion as Regulator of the Immune Response
By Mayo Clinic Staff
Immunologically mediated transfusion reactions have always been an important part of transfusion medicine. An additional use for blood transfusions has been investigated for several years now. Blood transfusions were previously predominantly regarded as a ‘substrate’ for immunologically mediated transfusion reactions, like in hemolytic transfusion reactions, or as a substrate for future immunologically mediated transfusion reactions, like in alloimmunization. Following the reports on the ‘blood transfusion effect’ on allograft survival, a new role for blood transfusions as immunomodulator or immunosuppressor has been discovered. Different aspects of the immunoregulatory effects of blood transfusions will be discussed
The immunological effects of blood transfusions have been of major consequence ever since blood was transfused. In the early days of transfusion medicine, hemolytic transfusion reactions made blood transfusions such a high-risk procedure that its use in humans was banned in several countries. When Karl Landsteiner discovered the ABO blood group antigens and their antibodies around 1900, the risk of hemolytic transfusion reactions was greatly reduced, and a major step towards modern transfusion medicine was taken [1]. With the reduced incidence of hemolytic transfusion reactions and the progress made in blood storage and blood banking, more extended surgery was made possible, leading to the present scope of major surgery including resection of malignant tumors, organ transplantation and cardiovascular surgery
Where transfusion medicine was initially hampered by complications related to the transfused erythrocytes, nowadays leukocytes and leukocyte products seem to be responsible for the majority of complications. Repeated transfusion of blood containing allogeneic leukocytes can lead to the formation of alloantibodies. It was already shown in the 1960s that the presence of these antibodies correlated with accelerated allograft rejection [4]. It therefore came as a surprise when Opelz et al.
[5] reported that transfused patients had a superior graft survival compared to non-transfused patients. Reducing the leukocytes within the transfused blood by passing the blood over a filter reduced the incidence of alloantibody formation, but also annihilated the positive effect on graft survival [6]. Leukocytes within a blood transfusion therefore seem not only able to activate the immune system, as with alloimmunization, but also to suppress the immune system, as seen with prolonged graft survival. This effect became known as TRIM (transfusion related immunomodulation) [7]. Where immunosuppression can be advantageous in a transplantation setting, it could have detrimental effects in other situations. The possibility of immunosuppression following leukocyte-containing blood transfusions led to several important questions [8]. Would transfusions increase the incidence of post-operative infections? Would they hamper immunosurveillance against cancer cells leading to more cancer recurrence following cancer surgery? What factor is responsible for the immunosuppressive effects? Are cytokines that are produced by the leukocytes during storage responsible, or is it microaggregates that are formed during storage. Or are viable functioning leukocytes needed? All these questions have resulted in many studies investigating various parts of this topic. Some (partial) answers have been found while other questions still remain unanswered.
Transfusion Products
Over the years, different types of erythrocyte products have been developed, and their use has differed between different countries. To make better usage of a scarce human source and to reduce the incidence of transfusion complications, most countries nowadays split the collected whole blood into different components. With the introduction of splitting the plasma from the red cells and transfusing the concentrated red blood
cells (RBCs), a reduction was seen in the occurrence of volume overload. Furthermore, the plasma remained available for further fractionation [9]. The removal of the buffy coat, containing 50–80% of leukocytes and over 90% of platelets, from the RBCs led to a reduction in post-transfusion febrile reactions [9]. Also, the buffy coat remained available to prepare platelet concentrates and/or be used in immunological research. To further reduce the incidence of especially HLA (human leukocyte antigen) alloimmunization in poly-transfused and/or transplant patients, filters were developed to selectively reduce the leukocytes in the blood (table 1). This lead to a reduction in the formation of a-HLA antibodies in these frequently transfused patients and further reduced the number of febrile complications [10, 11]. When analyzing the results from different studies across the world, it is important to realize that, although whole blood is not widely used anymore, all other types of erythrocyte products are considered ‘the’ standard erythrocyte product in some countries. Therefore, there is no ‘gold standard’ erythrocyte product that can be used as control in all studies.
Direct and Indirect Recognition
Following allogeneic blood transfusions, the recipient T cells recognize mismatched HLA molecules on transfused leukocytes as ‘non-self’. This direct recognition results in cytokine production (TNF-a, IL-1, IL-12) by recipient T helper (Th) cells, facilitating the proliferation and activation of cytotoxic T lymphocytes (CTLs) and antibody production by B cells [12]. One of the reasons for introducing changes, such as buffy coat removal or leukocyte reduction by filtration, in the manufacturing process of blood products was to reduce this direct recognition following blood transfusions [10]. Several randomized clinical trials have shown that, with patients requiring prolonged transfusion support, the alloimmunization frequency is indeed the lowest when filtered leukocyte-reduced blood products are used [13–15]. However, for patients who only undergo a single transfusion episode, this clinical advantage of filtered leukocyte-reduced blood products is not yet established [16]. Besides the possible absence of a clinical advantage in these patients because of the lack of recurrent exposure, another explanation may be that far less studies have been performed in these patient groups.
Following allogeneic blood transfusions, the HLA class II molecules on the recipient antigen-presenting cells (APCs) will bind fragments of transfused alloantigens. These are then presented to recipient CD4+ T-cells which recognize them through their T cell receptor (TCR) and start cytokine production, again facilitating the proliferation and activation of CTLs and antibody production by B cells. This indirect pathway of recognition is less effective than the direct pathway. Nevertheless, it may play a major role in some immunological effects of blood transfusions, like in the transplantation setting [17].
Immunological Transfusion Reactions
Donor against Patient
Transfusions containing plasma will also contain antibodies produced by the donor. Donor immunoglobulins directed against recipient leukocytes may cause non-cardiogenic lung edema, or TRALI (transfusion-related acute lung injury), the second most frequent severe transfusion complication. IgG antibodies directed against HLA class I, HLA class II or granulocyte/monocyte antigens have been identified in TRALI [18]. Transfusion of these can result in granulocyte aggregation, activation and microvascular pulmonary injury. With TRALI, by definition, respiratory distress occurs within 6 h after transfusion of a blood product containing as little as 30 ml of plasma. With appropriate respiratory intervention, most patients recover within 96 h of the original insult, without permanent pulmonary sequelae [19].
Strong anti-RBC antibodies (A, B or ‘irregular’ antibodies) in plasma transfusions can result in severe hemolysis. After multiple minor ABO-incompatible platelet transfusions (O to A or B), the transfused antibodies may produce a positive direct antiglobulin test, but hemolysis seldom occurs. Occasionally, anti-RBC antibodies in intravenous immunoglobulin (IVIG) can cause severe hemolysis.
When transfused immunocompetent donor T cells proliferate and attack recipient cells, transfusion-associated graft-versushost disease (TA-GVHD) develops. This rare complication is only seen in patients incapable of initiating an effective immune response against the transfused cells, and comes with a mortality rate of over 90% [20, 21]. Besides immunocompromised patients, other risk factors include the use of relatives as donor, HLA-homozygous donors and fresh (whole) blood containing viable lymphocytes [22, 23]. The chance of an HLA-homozygous blood product, haploidentical with the recipient, is approximately 1:800, but only a small minority of these transfusions actually cause TA-GVHD. Since in AIDS patients, TA-GVHD seldom occurs, there may be an additional role for T cell help by recipient CD4 cells [24].
Patient against Donor
Patients may, as a result of previous transfusions or pregnancies, have developed antibodies directed against components
of the transfused blood. Antibodies against transfused cellular components will result in accelerated destruction/removal of these transfused cells. Destruction of erythrocytes by antiABO or anti-Rh-D is nowadays seldom seen and most often the result of clerical or clinical errors [25]. Antibodies against other, ‘irregular’, antigens are found in 1–2% of unselected hospital patients, and are highly dependent on the number of previously received blood transfusions. 3–8% of patients will make new or additional antibodies against erythrocytes following a single transfusion episode [16, 26–28]. There is no standard time course of the titers of these new antibodies. They differ with the different type of antibodies and with the use of different detection techniques. Some are rapidly detected and become undetectable after only a few weeks while other antibodies take longer to become detectable but remain detectable for a longer period of time [29, 30]. This means that the specificities of antibodies that are found following transfusions partly depend on the time interval between transfusion and testing, and the test technique used [31]. As memory cells still remain after antibodies have been formed and later declined to undetectable levels, a following exposure to the antigen will result in rapid antibody production, eventually leading to a delayed hemolytic transfusion reaction [32]. Following 20 transfusions, the prevalence of antibodies against erythrocytes will have been increased to around 10% of patients, stabilizing at ±30% of patients following more than 100 transfusions [33]. The most important of the ‘irregular’ antigens, based on immunodominance and antigen frequency, are c, E and Kell. The preventive matching for c, E, and Kell in patients requiring chronic transfusion support will reduce broad red cell alloimmunization.
HLA antibodies, granulocyte antibodies, or a combination of both can cause the destruction of transfused leukocytes. This destruction may result in febrile non-hemolytic transfusion reactions (FNHTR). As leukocytes are only contaminants in most transfusion products, different techniques have been developed to reduce the leukocyte content of blood transfusions. Following the introduction of buffy coat removal, a decline in the incidence of FNHTR was seen [9]. Further reduction of the leukocyte content by filtration also further reduced the incidence of FNHTR [10, 11].
Antibodies against platelets may seriously reduce the life span of transfused platelets and eventually result in refractoriness to random donor platelets [34, 35]. HLA antibodies are most frequently involved in immunological refractoriness. Especially multi-specific HLA antibodies are associated with lack of increment [36]. In a minority of patients, HLA antibodies will disappear despite ongoing transfusion therapy. Occasionally, HPA antibodies can cause platelet refractoriness. These are nearly always found in combination with HLA antibodies [37]. The most frequently involved antigens are HPA-1a and HPA-5b. HPA antigens need processing and presentation by recipient APCs before antibody formation can be induced. As not all HLA class II molecules present HPA-1a and HPA-5b equally effectively, antibody responders to HPA are often restricted to specific HLA subtypes (HPA-1a with DR52a, HPA5b with DRw6) [38]. An additional rare transfusion complication, especially correlated with HPA-1a antibodies, is post-transfusion purpura (PTP). PTP occurs around 9 days (range 1–24 days) after a transfusion with whole blood, RBCs or platelets, and is mainly seen in women with previous pregnancies. They suffer bleeding and extreme thrombocytopenia as a result of autologous platelet destruction. Paradoxically, PTP patients are negative for the antigen against which the antibodies are directed. The precise mechanism is not understood, but following blood transfusion, recipient platelets may transiently express donor-derived antigens. ABO antibodies do very rarely cause platelet refractoriness, although a 10–20% reduced recovery of platelets can be seen with high titers [39].
Antibodies directed against non-cellular transfusion components may also result in transfusion reactions. Skin reactions are often related to IgE antibodies while IgG antibodies against plasma proteins, such as IgA or Chido/Rodgers, may result in severe hypotension and respiratory distress [40–42].
Mechanisms of Immunomodulation
Different mechanisms may be involved in the immunological effects of blood transfusions: i) the transfusion of soluble HLA class I peptides, donor immunoglobulins and other modulatory constituents of allogeneic plasma [43–47], ii) the transfusion of response modifiers, such as histamine, myeloperoxidase and soluble Fas ligand, produced and/or released during extracorporeal storage [43, 48–50], and iii) the transfusion of immunologically active allogeneic leukocytes that interact with recipient cells [51–53]. These 3 mechanisms may all play various roles in different immunomodulatory effects of blood transfusions. We will focus on the effects of blood transfusions on transplant survival, post-operative infections and cancer recurrence.
Transplant Survival
The initial report on the beneficial effect of blood transfusions on graft survival was published in 1973 by Opelz et al. [5]. Notwithstanding the progress made in immunosuppressive drugs and histocompatibility matching since then, the effect can still be demonstrated [54]. Important observations concerning the underlying mechanism were made by Persijn et al.
[6] who showed that a single transfusion could suffice as long as it was not leukocyte-depleted by filtration, and by Lagaaij et al. [55] who showed a crucial role for HLA-DR matching of the blood transfusion with the recipient. Over the years, mechanisms like donor selection, anergy and apoptosis have been suggested for this ‘blood transfusion effect’:
- Donor selection: Selection of a cross-match-negative donor in poly-transfused patients will preferentially select donors against whom a patient cannot easily respond [56].
- Anergy: Donor APCs lose costimulatory molecules during storage. Following transfusion, these impaired APCs cannot supply the costimulatory signal to the recipient T cells which will then become anergic instead of activated [57].
- Apoptosis: During storage, soluble HLA, Fas and other modulators accumulate in the donor blood. Following transfusion, these molecules bind to recipient T cells leading to apoptosis instead of activation [58].
If the above suggested mechanisms do not explain the importance of sharing 1 HLA-DR between blood donor and recipient, the following suggestion does: regulatory T cells [59, 60]. What special circumstances occur when blood donor and recipient share 1 HLA-DR? Following the blood transfusion, donor APCs carrying both mismatched HLA and matched HLA-DR containing donor-specific peptides can induce a strong immune response. Part of the induced recipient T cells will be directed against ‘matched HLA-DR containing donorspecific peptides’ (fig. 1b). If the donor subsequently donated an organ for transplantation, a special situation arises. The T cells that, by direct recognition, do react with the graft will become activated, and start expressing HLA class II molecules. Part of their HLA-DR molecules will contain donorspecific peptides. As a result, the transfusion-induced recipient T cells will recognize, as their specific target, these cells now expressing ‘matched HLA-DR containing donor-specific peptides’. They will thereby down-regulate this, by direct recognition initiated, reaction against the graft (fig. 1c) [61, 62]. The indirect recognition of the graft will also be reduced. In indirect recognition, the recipient APCs present donorspecific peptides in HLA class II. In a normal situation, recipient Th cells may recognize these and start cytokine production, thereby facilitating the proliferation and activation of CTLs and antibody production by B cells. In this situation, the transfusion-induced T cells will recognize the recipient APCs presenting ‘matched HLA-DR containing donorspecific peptides’ as their specific target. These T cells will thereby also reduce the effect of indirect recognition, which will further down-regulate the immune response against the transplanted organ (fig. 1c).
There are 2 key points in this suggested mechanism: i) the sharing of one HLA-DR between blood donor and recipient, facilitating the induction of the recipient T cells directed against ‘matched HLA-DR containing donor-specific peptides’ that will control a future reaction, and ii) the sharing of donor-specific peptide(s) between blood donor and organ donor, which are not shared with the recipient. These donorspecific peptides may consist of fragments of HLA class I molecules or from any other polymorphic protein. This mechanism can therefore also explain prolonged graft survival in patients where the blood donor and the organ donor were not the same person and did not share HLA antigens [60].
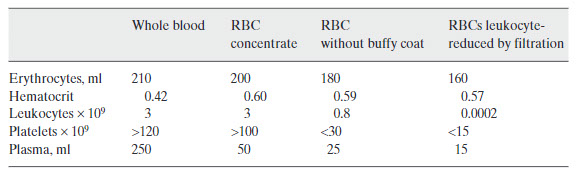
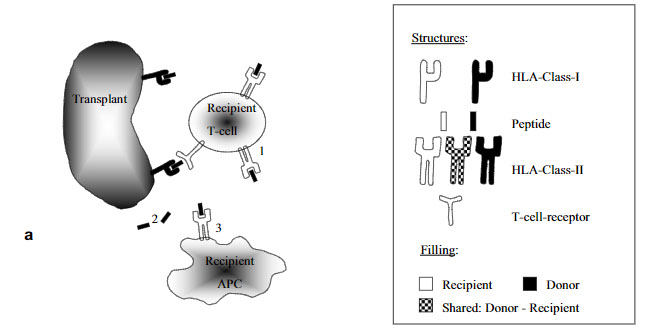
Fig. 1. Cellular interactions following blood transfusions and/or transplantation.
a Transplantation without previous blood transfusion.
1. By direct recognition activated T cells start expressing HLA class II.
2. Donorspecific peptides are released.
3. Recipient APCs present donorspecific peptides in HLA class II for indirect recognition.
Fig. 1b. Cellular interactions following blood transfusions and/or transplantation
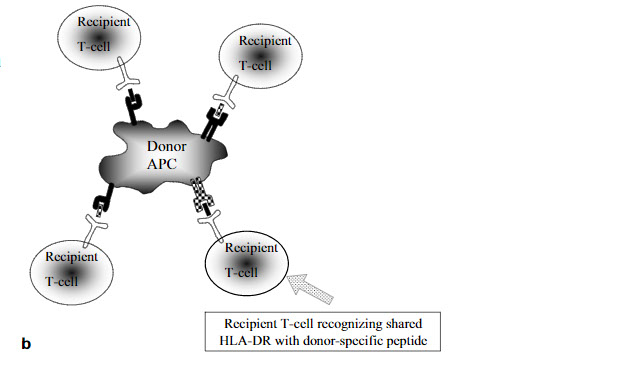
b Blood transfusion sharing one HLA-DR.A blood transfusion,sharing one HLA-DR,induces different T cells in the recipient. Part of these T cells will recognize the shared HLA-DR with donor-specific peptide
Fig. 1c. Cellular interactions following blood transfusions and/or transplantation
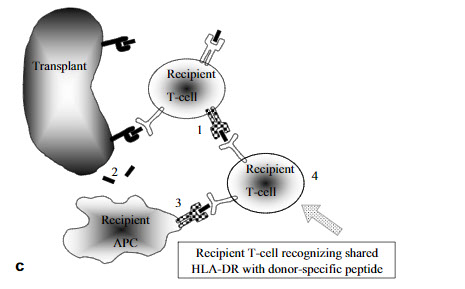
c Transplantation after an HLA-DR-shared blood transfusion.
1. By direct recognition activated T cells start expressing HLA class II.
2. Donor-specific peptides are released.
3. Recipient APCs present donor-specific peptides in HLA class II for indirect recognition.
4. TheT cells induced by transfusion will recognize APCs and activated T cells as specific target
Postoperative Infections
The effect of blood transfusions on the incidence of postoperative infections is widely researched [53, 63–70]. Of the possible underlying mechanisms, the effects of cellular components are the most extensively investigated in the perioperative setting, especially in abdominal surgery and cardiac surgery. Here, the shift towards a Th2 type of immune response by blood transfusions is superimposed on the shift in Th response associated with trauma and surgery. This induces impairment of monocyte and natural killer (NK) cell functions with reduced phagocytosis, reduced killing of microorganisms and an absent pro-inflammatory response to bacterial endotoxins as lipopolysaccharide (LPS) [71, 72]. The randomized controlled trials investigating postoperative infections within patient groups receiving blood products differing in leukocyte content, produced results that, at first glance, were contradictory [53, 66, 68, 73–77]. Part of this was based on the lack of a uniform definition to establish postoperative infections, making especially multi-center studies prone to confounding. Different studies showed effects ranging from transfusions having absolutely no effect, to transfusions being the number-one predictor of postoperative infections. More precise analyses of the data seems to suggest the existence of a threshold for the total number of leukocytes being transfused in a single transfusion event of about 3 ´ 109, above which an increase in postoperative infections may be seen. In studies comparing whole blood or plasma-reduced RBC transfusions with transfusions leukocyte-reduced by filtration, significant differences were found even after just 1 single transfusion [71, 74]. In studies comparing buffy coat-depleted RBCs with transfusions leukocyte-reduced by filtration, significant differences can be found in the subgroup of patients receiving more than 2–4 units of blood, depending on the efficacy of the buffy coat removal [53, 73, 77]. In some studies comparing buffy-coat depleted RBCs with transfusions leukocyte-reduced by filtration, the majority of patients in the non-filtered trial arm did not receive enough transfusions to reach the threshold, resulting in non-significant differences between the trial arms [75]. The ongoing trend to minimize the number of perioperative blood transfusions will increase the number of patients needed within a transfusion study to reach statistically sound conclusions. With the introduction of universal leukocyte reduction by filtration for all erythrocyte transfusions, reductions in febrile reactions and the use of antibiotics have been reported, while a reduction in serious infectious complications is seldom observed [78–80]. This lack of effect may be based on the unselected patient population analyzed in the before/after studies [81]. These include large groups of patients undergoing types of surgery for which a beneficial effect has never been shown or was never investigated
![]()
The immunocompromised patient and transfusion
https://pmj.bmj.com/content/77/906/230
Immunocompromised patients are usually seriously ill and many such patients, especially those undergoing stem cell transplantation, have prolonged periods of pancytopenia and consequently, heavy transfusion requirements.1 All transfusions are potentially hazardous but transfusions to immunocompromised patients cause additional problems, which may be immunological or infectious. This review describes these special problems and ways to alleviate them. It should be useful to anyone treating immunocompromised patients particularly specialists in haematology, transfusion medicine, infectious diseases, oncology, transplant surgery, anaesthesia, and neonatology.
Different parts of the immune system—either non-specific (phagocytes, complement, etc) or specific immunity (cellular or humoral) or combinations thereof may be affected. Patients with pure B cell immunodeficiency have few transfusion related problems. Both hereditary and acquired defects of the immune system occur (table1).2 Inherited defects requiring transfusions are rare while acquired causes are relatively common. Neonates weighing less than 1200 g are physiologically immunocompromised.3
Immunological hazards
Problems such as haemolytic transfusion reactions and HLA alloimmunisation leading to transfusion refractoriness are well known and common to all patients. Less well known (but of particular importance to immunocompromised patients) is transfusion associated graft-versus-host disease (TA-GvHD), mediated by donor derived, “passenger” T lymphocytes in cellular components, and immunomodulation that may increase the risk of infection and cancer recurrence.
TRANSFUSION ASSOCIATED GRAFT-VERSUS-HOST DISEASE
This has the same prerequisites as the GvHD that follows allogeneic stem cell transplantation, that is (a) immunocompetent donor T cells, (b) histoincompatibility between donor and recipient, and (c) inability of the recipient to reject donor T cells. It has been reported after stem cell (allogeneic as well as autologous) transplantation, after chemotherapy for acute leukaemia, in Hodgkin's disease, severe combined immune deficiency, and after neonatal and intrauterine transfusions.3-7 Neonates develop TA-GvHD especially if intrauterine transfusion is followed by postnatal exchange transfusion. It is believed that the intrauterine transfusion induces tolerance preventing the rejection of lymphocytes transfused subsequently.3 8 In aplastic anaemia, where there is usually no cellular immune deficiency, and in AIDS, where there is, no cases of TA-GvHD have been described.9 10 In the case of AIDS, it may be that donor T cells are themselves infected by HIV, preventing their engraftment.
Rarely, immunocompetent patients can also suffer from TA-GvHD. This may happen when donor and recipient share HLA antigens—particularly if the donor is homozygous for an HLA haplotype that the recipient is heterozygous for. Under these circumstances, the recipient has HLA antigens that are foreign to the donor but not vice versa (fig 1).
Donor homozygous for a HLA haplotype for which recipient is heterozygous.
The recipient therefore does not reject donor T lymphocytes that can recognise recipient HLA antigens as foreign and cause TA-GvHD. Three sorts of immunocompetent patients are prone to TA-GvHD: (a) patients receiving cellular components from close relatives, (b) patients in places such as Japan, where people often share a few common HLA haplotypes, and (c) patients receiving HLA matched components.11-14
Cellular components like red blood cells (RBC), platelet and granulocyte concentrates and also fresh liquid plasma, but not previously frozen components like fresh frozen plasma, can cause TA-GvHD.15 Fresh blood (<72 hours) is a significant risk factor because lymphocyte viability declines during storage.16 Thus, in addition to the immune status of the host and the degree of HLA similarity between blood donor and recipient, TA-GvHD depends on the number and viability of lymphocytes transfused. The minimum dose of lymphocytes required for TA-GvHD was estimated to be 1 × 107/kg but TA-GvHD has been described even with filtered blood components which result in doses of 2–5 × 104 lymphocytes/kg. Hence, the quality and minimum dose of lymphocytes for TA-GvHD to develop remains uncertain.16TA-GvHD presents four to 30 days after transfusion and can develop after even a single unit.
For reasons that are unclear, TA-GvHD is more severe than that occurring after allogeneic stem cell transplantation. Skin (erythematous maculopapular rash, which may progress to generalised erythroderma and bullae), gastrointestinal tract (diarrhoea) and liver (raised liver enzymes and bilirubin) are involved. In addition, fever, lymphadenopathy, and suppression of host haematopoiesis by donor T cells (reducing immunity further and causing thrombocytopenia and anaemia) commonly occur.17 Thus, TA-GvHD is easily confused for problems such as infection or treatment related toxicity in immunocompromised, ill patients and the diagnosis may be missed. Histological features of lesional tissues are characteristic and similar to those seen in classical GvHD. Conclusive diagnosis requires demonstration of HLA or sex chromosome chimerism. Treatment methods are similar to that for GvHD in other situations: high dose corticosteroids (for example, methyl prednisolone 1 g/m2 followed by rapid taper), cyclosporin (for example, 6 mg/kg intravenously 12 hourly on alternate days) and sometimes, antilymphocyte globulin or anti-T cell antibodies. Supportive treatment (platelet and RBC transfusions, granulocyte colony stimulating factor, antibiotics, etc) may also be required. Mortality is nearly 90% despite treatment.4Since treatment is so ineffective, it is important to prevent TA-GvHD from occurring.
TA-GvHD can be prevented in susceptible patients by avoiding unnecessary transfusion, careful donor selection and by inactivating lymphocytes with γ-irradiation or ultraviolet-B (UV-B) light. Current leucocyte filters, though capable of reducing total leucocyte numbers by >3 log10 (>99.9%), fail to prevent TA-GvHD because lymphocytes are not sufficiently reduced. Being affinity filters, cellular characteristics other than size, such as surface tension, adhesion and activation, also determine what cells are retained.18 γ-Irradiation of cellular blood components to minimum doses of 2500 cGy (25 Gy) to the mid-plane of the container and 1500 cGy to all other parts is used to prevent TA-GvHD.19 This prevents 14C-thymidine incorporation by lymphocytes after mitogenic stimuli. A 500-cGy dose may suffice to prevent the physiologically relevant proliferation in mixed lymphocyte culture.20 Doses <5000 cGy do not affect RBC, platelet, or granulocyte function and survival adversely.21 Dedicated blood irradiators (containing a shielded 137caesium source) or conventional facilities may be used.22 Irradiation of an RBC unit or of six units of platelets takes around two minutes. The delivered dose is a function of the residual radioactivity of the source and time of exposure. With time, exposure needs to be increased to achieve the required dose. Irradiated cellular components (other than stem cell grafts and donor lymphocyte infusions given for a graft-versus-tumour effect) are used for the following categories of patients (box 1).
An alternative to γ-irradiation is exposure to UV-B light (280–320 nm), which abolishes the capacity of lymphocytes to respond as well as to stimulate. This is potentially simple and inexpensive but the equipment is not readily available and it is difficult to ensure uniform UV exposure. Furthermore, standard blood bag plastic is opaque to UV light, requiring the use of special bags.24 UV-B for the prevention of TA-GvHD is still experimental.
Box 1: Patients who should receive irradiated cellular components
Established indications
Postallogeneic stem cell transplant when absolute lymphocyte count is <0.5 × 109/1.19
Some immunodeficient patients.19
Intrauterine transfusions.19
Transfusions from close relatives.19
Doubtful indications
Patients with chronic GvHD.23
Postautologous stem cell transplantation.23
Patients with malignancies when absolute lymphocyte count <0.5 × 109/l.19
Patients with AIDS.10
Neonates <1200 g.23
Recipients of HLA matched cellular blood components.23
IMMUNOMODULATION
This poorly understood phenomenon is believed to be caused by transfused leucocytes leading to a decrease of T and B lymphocytes, natural killer cells, and monocytes.25 Immunomodulation is reported to increase haematological and non-haematological tumour recurrence (though this is challenged; see below), and infection after surgery.24-26 27 Pre-storage leucodepletion (see below) may reduce this problem, but there is no consensus on this issue.28 29
Infectious hazards
All blood donations are screened for infections such as hepatitis B and HIV that are dangerous to all transfusion recipients—immunocompetent or otherwise. But agents such as cytomegalovirus (CMV) that cause few problems in immunocompetent individuals can cause serious disease in immunocompromised patients.
CYTOMEGALOVIRUS INFECTION
This widespread herpes virus is often acquired perinatally or in childhood. Seropositivity rates in apparently healthy adults are 30% to 80% in developed countries and nearly 100% in developing countries.30 In immunocompetent subjects a mild or subclinical infection is caused, which may persist in latent form in leucocytes. Because of the high prevalence, blood donations are not routinely tested for CMV. In immunocompromised patients, CMV can cause a severe, disseminated infection resulting in interstitial pneumonitis, hepatitis, retinitis, enteritis, and encephalitis.31 CMV causes tissue injury directly as well as by non-cytopathic means, where CD8+ cytotoxic T lymphocytes lyse cells displaying viral antigens in conjunction with HLA class I molecules.32 The risk of acquiring infection is proportional to the number of donor exposures.
CMV transmission can be prevented either by using blood from CMV negative donors or by leucodepleting cellular components.33 34 35 Screening tests for CMV involve the detection of specific IgM or IgG antibody by enzyme linked immumosorbent assay. IgM indicates an acute infection and IgG, past exposure. CMV positive individuals have either anti-CMV IgG or IgM and negative individuals neither. Only 3%–12% of CMV positive donors may be able to transmit CMV.35 It was suggested that anti-CMV IgM positive (+/-IgG) donations are more infectious than IgG positive, IgM negative ones but this remains unproved.10 Other methods of CMV diagnosis are viral culture, antigen detection, shell vial assay, and polymerase chain reaction. These are useful in patients but not blood donors. In CMV positive, immunocompromised patients, reactivation of latent, endogenous infection is more common than transfusion derived infection. Hence, such patients and CMV negative recipients of CMV positive stem cell or organ grafts are not usually given CMV negative components.36
Third generation leucocyte filters remove neutrophils and monocytes efficiently without excessive RBC or platelet loss. Filtered components are equivalent to CMV negative donations if residual leucocytes are <5 × 106 per RBC unit or adult therapeutic dose of platelets.30 Leucodepletion for selected patients means that an inventory of CMV negative donors is unnecessary—a particular advantage in many developing countries where the availability of seronegative donors (and the demand for seronegative blood) is small. But, before advocating expensive filters for this purpose, studies on the natural history of CMV infection in immunocompromised patients in these countries are needed. The advantages of leucodepletion are listed in box 2.
Box 2: Advantages of leucodepletion
CMV transmission.
HLA alloimmunisation in multiply transfused patients.37 38
Some febrile non-haemolytic transfusion reactions.37 39
Human T cell leukaemia virus transmission.40
Epstein-Barr virus transmission.40
Bacterial infection.37 41
Tumour recurrence.28 37
TA-GvHD.15
Some transfusion associated lung injury.37 42
The levels of leucodepletion required for preventing HLA alloimmunisation and FNHTR are 5 × 106 and 5 × 108 respectively.19 Potential multitransfusion patients (such as transplant patients and those undergoing treatment for malignancies) should receive leucodepleted components. Separate filters are used for RBC and platelets. Pre-storage filtration is better than post-storage (laboratory) or pre-transfusion (bedside) filtration for at least three reasons. Firstly, it is less cumbersome, better controlled, and has fewer failures.43 Secondly, cytokine release by leucocytes is prevented and this may reduce febrile non-haemolytic transfusion reaction.44 45 Thirdly, leucocytes are removed before they can disintegrate and release free virus such as CMV into the plasma.46 Disadvantages of leucodepletion include cost, time, increased leukaemia relapse due to the loss of the graft-versus-leukaemia effect (though this is challenged; see above) and occasional hypotensive reactions, possibly due to plasma-protein activation and bradykinin release.47 48
The following categories of patients (box 3), but not patients undergoing non-myeloablative chemotherapy, may need CMV negative or leucodepleted cellular components.49 Obviously, stem cell transplants, donor lymphocyte infusions, and granulocyte concentrates must never be leucofiltered!
Box 3: Patients who may need CMV negative or leucodepleted cellular components
Established indications
CMV negative recipients of CMV negative stem cell allografts.
CMV negative recipients of CMV negative organ allografts.
CMV negative AIDS patients.
CMV negative patients with inherited immunodeficiencies.
CMV negative pregnant women.
Fetuses needing intrauterine transfusion.
Neonates <1200 g with a CMV negative mother.
Doubtful indications
CMV negative stem cell autograft recipients.
CMV negative patients undergoing splenectomy.
Leucodepletion by other means such as centrifugation, washing, freezing, and thawing may be insufficient to prevent CMV transmission. γ-Irradiation cannot be used because the dose needed to inactivate the virus can damage blood cells.50 Other methods are used to prevent overt CMV infection (exogenous or reactivation) in allogeneic stem cell transplant patients.51 These include, (a) weekly surveillance cultures from day 30 to 100 post-transplant, (b) pre-emptive gancyclovir, if surveillance cultures are positive, and (c) acyclovir and intravenous IgG from day 7 to 100 post-transplant. CMV infections in immunocompromised patients are usually treated with gancyclovir and CMV immune globulin but mortality is high, especially with CMV interstitial pneumonitis.
OTHER INFECTIONS
Immunocompromised patients are also prone to other infections—viral, bacterial, fungal, and protozoal. For instance, allogeneic stem cell transplant patients often have a characteristic sequence of infections (fig 2) but such infections are often either endogenous or have portals of entry other than transfusion.51 Obviously, infected units can cause serious disease, particularly in neutropenic and splenectomised patients. Non-leucoreduced, allogeneic cellular components, by causing “immunomodulation” (see above), may exacerbate infection.
Figure 2
Download figure
Open in new tab
Download powerpoint
Figure 2
Immune deficiency and infection hazards after allogeneic stem cell transplantation (CMV = cytomegalovirus; HSV = herpes simplex virus; VZV = varicella zoster virus).
Other common herpes viruses including Epstein-Barr virus (EBV) and the human herpes viruses (HHV) 6, 7, and 8 are also important in this setting. EBV like CMV causes a mild, self limited infection in immunocompetent subjects. EBV is latent in B lymphocytes and can be transmitted through cellular blood components but this is uncommon because of the presence of neutralising anti-EBV antibodies in the donation itself. Only if the units transfused are exclusively from a donor who does not have such antibodies, can post-transfusion EBV infection occur—and then, only rarely in immunocompetent patients in whom EBV specific, cytotoxic T lymphocytes prevent uncontrolled B lymphocyte proliferation. In immunocompromised patients, post-transfusion EBV infection can lead to EBV associated lymphomas.52
Box 4: Summary and learning points
Immunocompromised patients receive more transfusions.
Tranfused leucocytes cause special problems.
TA-GvHD is caused by donor T cells.
Irradiating cellular components prevents TA-GvHD.
Leucocytes cause immunomodulation increasing infection and tumour recurrence.
Leucodepletion reduces problems due to immunomodulation.
CMV latent in leucocytes can cause disseminated infection.
CMV negative blood or effective leucodepletion prevent CMV transmission.
EBV may cause B cell lymphoma and parvovirus B19 can affect haemopoiesis.
Pre-storage is better than post-storage leucodepletion.
HHV 6–8 are also lymphotropic and have biological and epidemiological similarities to CMV including latency.53 Hence, transmission through transfusion is possible. The rare reports of serious infections with these viruses in immunocompromised patients suggest that they were reactivations of latent infection. It is uncertain if HHV seronegative, immunocompromised recipients need HHV negative transfusions.54
Some immunocompromised patients may have pure red cell aplasia due to persistent infection with parvovirus B19, which is transfusion transmissible, particularly through coagulation factor concentrates.55 This has been reported in patients with AIDS, Nezelof's syndrome, and in children in remission after treatment for acute lymphoblastic leukaemia.56-58 Thrombocytopenia may also occur.59 Infection is treatable with immunoglobulin infusions. The parvovirus B19 seropositivity rate among blood donors is 30%–60% but many probably merely represent past exposure. Donors capable of transmitting the infection are estimated to be only about 0.03% and it is not clear if, when, and how donations need to be screened
![]()
Therapeutic Plasma Exchange as a Treatment for Autoimmune Neurological Disease
https://www.hindawi.com/journals/ad/2020/3484659
Introduction. Therapeutic plasma exchange (TPE) is commonly used as treatment of certain autoimmune neurological diseases (ANDs), and its main objective is the removal of pathogenic autoantibodies. Our aim was to describe the clinical profile and the experience with the usage of TPE in patients with ANDs at our institution. Methods. This is an observational retrospective study, including medical records of patients with diagnosis of ANDs who received TPE, between 2011 and 2018. Characteristics of TPE, such as number of cycles, type of replacement solution, and adverse effects, were evaluated. The modified Rankin Scale (mRS) was applied to measure the clinical response after the therapy. Results. 187 patients were included with the following diagnoses: myasthenia gravis (MG), n = 70 (37%); Guillain–Barré syndrome (GBS), n = 53 (28.3%), neuromyelitis optica spectrum disorders (NMOSD), n = 35 (18.7%); chronic inflammatory demyelinating polyneuropathy (CIDP), n = 23 (12.2%); and autoimmune encephalitis (AE), n = 6 (3.2%). The most used types of replacement solution were albumin (n = 131, 70%) and succinylated gelatin (n = 45, 24%). All patients received a median of five cycles (IQR 5-5). Hypotension and hydroelectrolytic disorders were the main complications. After TPE, 99 patients (52.9%) showed improvement in the mRS scores and a statistical significance () was seen between the admission score and after TPE for every diagnosis except for CIDP. Conclusion. TPE has an adequate safety profile, and improvement in functionality in treated patients reflects its effectiveness.
1. Introduction
Therapeutic plasma exchange (TPE) is defined by the American Society for Apheresis (ASFA) 2019 guidelines as “A therapeutic procedure in which the blood of the patient is passed through a medical device which separates plasma from the other components of blood….”. Unlike plasmapheresis, TPE involves plasma removal and replacement with a solution such as a colloid solution (e.g., albumin and/or plasma) or a combination of a crystalloid/colloid solution [1].
Even though it was initially conceived as a treatment for hematological diseases with presumed or demonstrated immune pathophysiology [2], treatment using TPE has been extended to a variety of pathologies including kidney, autoimmune rheumatological, and neurological diseases, with the latter being the pathology most frequently treated by TPE [3]. The increased usage of TPE has likely followed an increased understanding of its mechanisms of action, which range from the removal of pathogenic autoantibodies and immune complexes to improvement in monocyte function [2]. Among the autoimmune neurological diseases (ANDs) treated using TPE, chronic inflammatory demyelinating polyneuropathy (CIDP), Guillain–Barré syndrome (GBS), myasthenia gravis (MG), neuromyelitis optica spectrum disorders (NMOSDs), and autoimmune encephalitis (AE) are well described. TPE is typically used alone or in conjunction with other treatment options, such as intravenous immunoglobulins (IVIG) and corticosteroids, as a first-line treatment for some of these disorders.
The great majority of descriptions of the clinical experience of TPE usage for this group of diseases are from North American or European cohorts [4–7]. In addition, environmental and genetic factors differ between populations, and these factors may influence the response the individuals have on different therapeutic interventions. Many of the mentioned ANDs have shown an exponential increase in occurrence in recent years [8–11]. Therefore, it is important to study in depth the safety and effectiveness of their available treatments, such as TPE. In this study, we aimed to describe the factors associated with indications, types of replacement solution, adverse effects, and treatment effectiveness of this intervention in patients with different ANDs.
2. Material and Methods
This was a retrospective and observational study conducted at Fundación Valle del Lili, a high-complexity center in Cali, Colombia. The medical records of patients with ANDs diagnosed by neurologists, who received at least one TPE in our institution between 2011 and 2018, were included in our analyses. In each case, the number of treatment cycles, type of replacement solution, clinical characteristics as duration of inpatient stay, adverse effects, and death of patients were evaluated. For identifying relapses, admissions due to ANDs up to one year after TPE were quantified.
TPE was prescribed by neurologists and was always administered in the intensive care unit (ICU) by trained nurses. The procedure comprised centrifugation, where prophylactic calcium was not routine. In each case, according to TPE characteristics (e.g., volume, replacement solution), anticoagulation with citrate and replacement solutions were administered in the proportions of 1 : 12 to 1 : 16.
Since most of the evaluated diagnoses (e.g., MG, GBS, and CIDP) have a disease-specific scale, we used the modified Rankin Scale (mRS) to measure and compare the clinical improvement between our patients. Although initially used for stroke treatment, it has also been applied in several different contexts including the evaluation of outcomes in NMOSD [12], AE [13, 14], MG [15], CIDP [16], GBS [17], and other neurological conditions [18].
Thus, the mRS was applied to every patient at three different times: at admission, using the admission note of the department of neurology; after TPE, using their progress note registered after completing all the cycles; and 90-days after receiving TPE, using medical records of follow-up appointments with neurologists. mRS measurement was performed by the research group with a previous training by a neurology resident.
The mRS was first introduced in 1957 for measuring outcomes in acute stroke and was modified to its seven-grade version as follows: 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, dead [19, 20]. The proposed interpretation of the mRS is based on two ranges of scores, 0–2 and 3–6, which are defined as favorable and nonfavorable, respectively; however, when looking at scores as individual points, changes in a single one have been stated to be clinically relevant [21].
Statistical analyses were performed using STATA v.14® (StataCorp LP, College Station, TX). Quantitative variables are shown as means and medians with standard deviations (SD) and interquartile ranges (IQRs) according to their distribution on the basis of the Shapiro–Wilk test. Quantitative variables are also shown with frequencies and proportions. mRS scores are presented as medians with IQRs; scores at admission and after TPE were compared with the Wilcoxon matched-pair signed-rank test.
3. Results
A total of 187 patients were included in this study. Most of them were women (n = 104, 55.6%), and the median age at admission was 50 (IQR 32–64) years. The most frequent AND was MG, with 70 cases (37.4%), followed by GBS, NMOSD, CIDP, and AE (Table 1).
![]()
Table 1
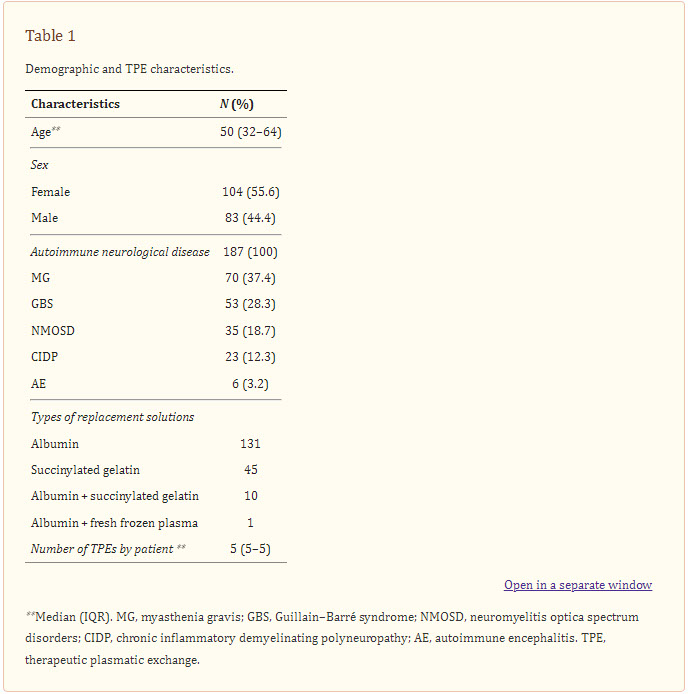
Demographic and TPE characteristics.
TPE was performed using the centrifugation method, and a catheter was placed at the jugular (n = 166, 88.7%), femoral (n = 18, 9.6%), or subclavian (n = 3, 1.6%) positions.
The replacement solutions used in TPE were albumin, fresh frozen plasma, and succinylated gelatin. Albumin alone was used in most patients (n = 131, 70.5%), whereas albumin in combination with fresh frozen plasma was used in one patient only. In the general sample, the median number of TPEs per patient was five (IQR 5-5) (Table 1). Separated by diagnosis, it was the same in MG, AE, and CIDP; in NMOSD and GBS, the median remained five with a range slightly wider (IQR 5-6). All of the TPEs were indicated daily. The mean exchanged volume was 2278 mL (SD 525.7), and the mean TPE time was 67 minutes (SD 17.5).
Patients had a median of inpatient stay of 12 (IQR 8–19) days. Those with AE had the longest of all, with a median of 24.5 (IQR 17–49) days; this group also required more days in the ICU than other groups. The complications observed after TPE was provided were as follows: 106 (56.6%) patients presented with hypotension, 102 (54.4%) presented with electrolytic disorders, and 85 (45.4%) presented with some complications associated with the Mahurkar catheter (other complications were found at a lesser extent). The presence of infection was evaluated during the 14 days following TPE: a total of 21 (11.2%) cases were observed, of which the majority were patients with GBS (n = 10, 47.6%). Infections were subdivided on the basis of the causative agent, with bacteria as the principal factor (n = 17/21, 80.9%); viral infections occurred in two (9.5%) patients, fungal infection in two (9.5%), mycobacterial infection in one (4.8%), and parasitic infection in one (4.8%). The most common pathogen was E. coli, with 4 cases (19%), followed by P. mirabilis and S. aureus, both with 3 cases (14.3%). Two individuals had two agents of infection at the same time (Table 2).
Table 2
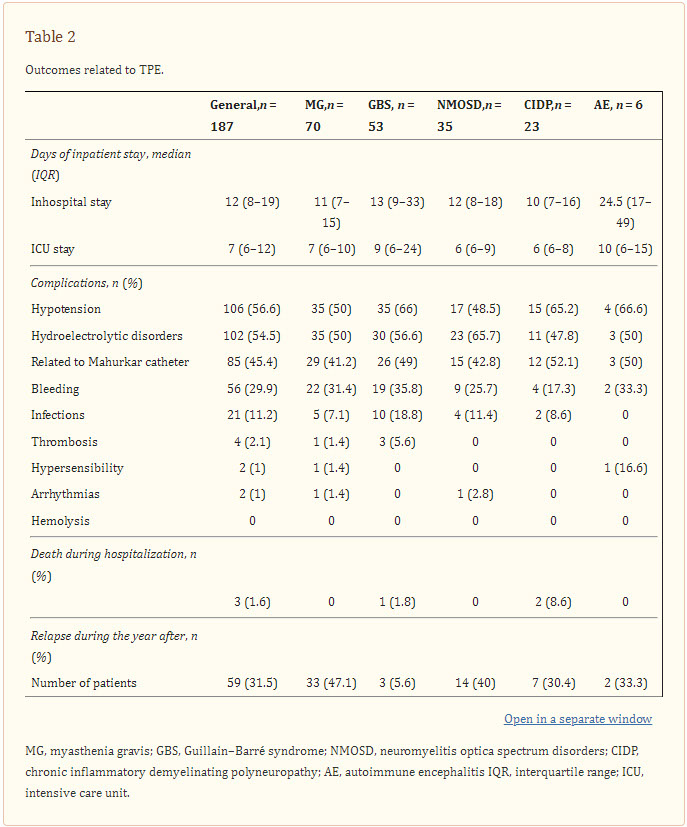
Outcomes related to TPE.
Three (1.6%) patients died during hospitalization due to causes associated with their neurological disease and septic shock. Clinical relapse in the year following TPE was measured, and the largest proportion of relapses occurred in patients with MG (Table 2).
On the basis of the mRS score for each patient at admission and after TPE, 99 (52.9%) patients showed decreased scores after TPE, 67 (35.8%) showed no difference, and 21 (11.2%) showed increased scores after therapy. Although three patients died, two were receiving TPE at the time of death and the other one had finished TPE and remained alive for three additional months, which explains the two scores of “6” in the final measurement (Table 3). In addition, a statistically significant difference was noted between the median mRS scores before and after TPE in general () and for each disease: MG, ; GBS, ; NMOSD, ; AE, . CIDP was the only one in which the difference was not significant () (Table 4).
Table 3
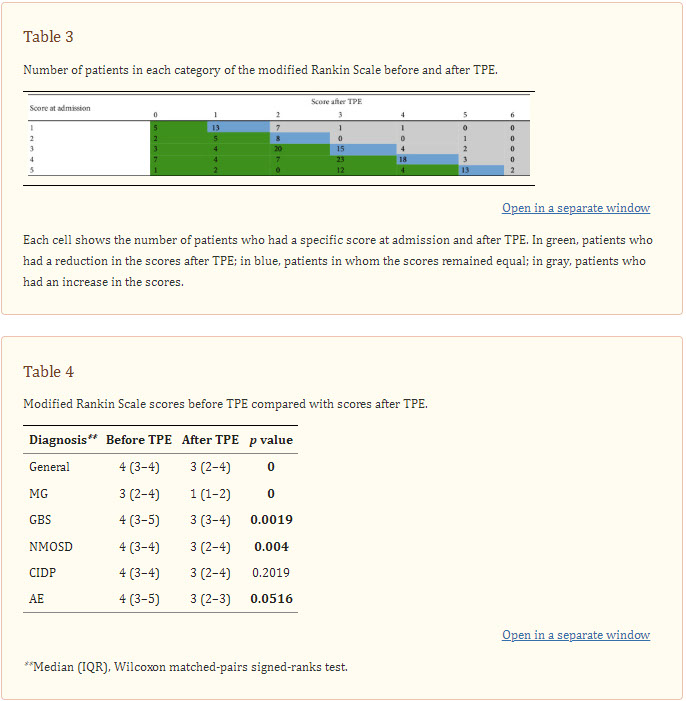
Number of patients in each category of the modified Rankin Scale before and after TPE.
Table 4
Modified Rankin Scale scores before TPE compared with scores after TPE.
We calculated the mean/median time patients took to present relapses depending on their mRS after TPE and 90 days after TPE. It is important to notice that only 104 patients have a calculated mRS 90 days after TPE due to absence of medical records in that specific time (Table 5).
Table 5
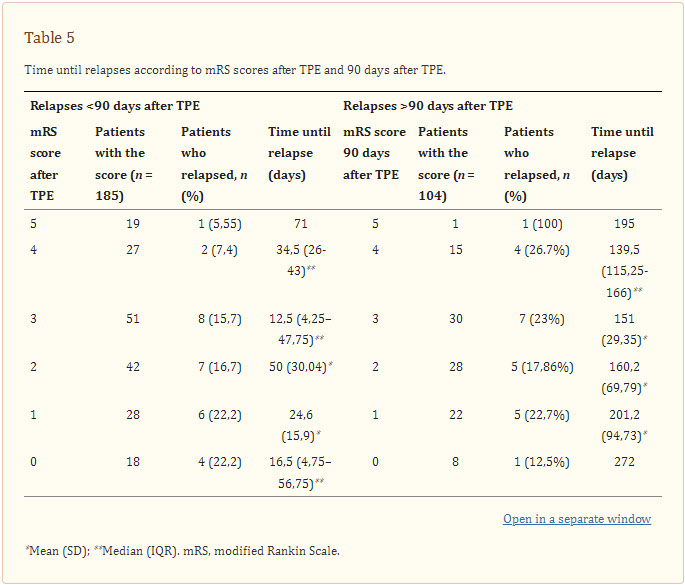
Time until relapses according to mRS scores after TPE and 90 days after TPE.
On the other hand, we calculated the difference in points between the mRS before and after TPE (the “Delta TPE”) and looked for any interrelation with relapse time, but we did not find any.
4. Discussion
This is a real-life study that shows the experience with the usage of TPE in multiple ANDs in a big cohort of 187 patients who were treated at a high-complexity center in Colombia. In 2019, the ASFA guidelines [1] defined specific recommendations for the use of TPE, and GBS, CIDP, MG, and NMOSD were included among the diseases of interest. Of these diseases, the strongest grades of recommendation were assigned to MG and CIDP, whereas the weakest grades were assigned to NMOSD and GBS [1]. Furthermore, neurological conditions in general are known to account for 44% of the cases in which TPE is indicated [22].
As previously discussed, TPE is as effective as IVIG in the treatment of GBS, CIDP, and MG; therefore, the decision to choose one or another treatment depends, in most cases, on the clinician’s experience, treatment availability, and expenses. What can vary is the scheme in which the treatment is provided upon diagnosis [23]. In the present study, TPE was indicated in every patient due to the severity of their disease or therapeutic failure of other therapies, with MG being the most frequently observed disease (similar to previous reports) along with GBS [22]. In our center, TPE, rather than IVIG, is the preferred management option for ANDs, and it achieves favorable results.
Generally, the number of treatment cycles was approximately five, consistent with descriptions that report a total fluid exchange divided five times in MG [24], GBS [22], and CIDP [3, 25]. Jiao et al. reported a slightly different range, 2–7 treatment cycles, in 29 patients with NMOSD [26], and Moser et al. reported a wider range of cycles, 3–13, in 12 patients with AE [27].
In the analyses of the complications, hypotension occurred in approximately half of our patients even though a replacement solution was provided with TPE to prevent this outcome; this differs to apheresis where there is no fluid replacement [2]. Nonetheless, hypotension is common when using TPE for different indications [27–31]. Hydroelectrolytic disorders were the second-most frequent adverse reaction, not only in our study but also in the study by Clarck et al., who found that 59 of their patients required the replacement of at least one electrolyte after TPE (primarily for potassium and magnesium abnormalities) [30]. In our patients, 11.2% presented with infections, of which 80.9% were caused by bacteria. This finding is similar to a group studied by Lemaire et al., who reported infections caused primarily by bacteria, followed by viruses and fungus [29]. Severe adverse reactions, such as hypersensibility reactions and arrhythmias, were seen at a lower frequency in our group.
Lemaire et al. reported 50 patients who were admitted to the ICU, with 40% admitted due to neurological causes. In their cohort, the median inpatient stay was 20 (IQR 12–35) days, which was similar to our findings (20 days, IQR 8–19), whereas they reported a mortality proportion of 8% compared with 1.6% in our group [29]. As has been previously stated, two of the deaths in our study occurred before TPE was completed; thus, in some cases, these treatment options are given to critical patients in whom a clinical response may be difficult to achieve [30].
Clinical responses after TPE have been measured in different ways among several studies. For instance, Morgan et al. clinically evaluated the improvement in patients with NMO, concluding that there was a moderate improvement in functional scales [32]. For MG, CIDP, and GBS, Momtaz et al. focused on muscle strength, finding that of 31 patients with MG, 83.8% achieved a complete response; of 8 patients with CIDP, 87.5% achieved such a response; and of 39 patients with GBS, 51.3% responded in this way [25]. Titulaer et al. described a cohort of 577 patients diagnosed with AE, of whom 461 received first-line therapies, including TPE: 97% maintained mRS scores of 0–2 during a year of follow-up [33].
In this way, what we identify as the strength of our study is that we could compare multiple ANDs and bring objective data regarding clinical improvement after TPE with the application of the mRS, based on the experience of different authors who evaluated these diseases separately. It is to remark that although mRS is not a disease-specific scale for the diagnoses of interest, it is one that allows an approach in a more unified way among them. Then, similarly to the studies previously mentioned, 52.9% of our patients showed an improvement in their mRS scores whereas 35.8% did not show any response to treatment. Although no significant difference was noted between mRS scores before and after TPE in patients with CIDP, a decrease in median scores for each disease was observed in all diagnoses.
We did not find a pattern regarding time until relapses in relation to the mRS scores, but in the patients with a score of “0” at 90-days after TPE, it took the longest period of time to present a relapse, as would be expected. Then, it is reasonable to think that other factors may be associated with a better outcome following TPE, and it may be useful to consider these when making treatment decisions or considering expectations. In NMOSD, these factors include anti-AQP4 seronegativity (patients who are seronegative for anti-AQP4 but seropositive for anti-myelin oligodendrocyte glycoprotein generally present better outcomes) [12] and combination treatments with immunosuppressants [34]. In GBS, additional factors include the number of exchanges on the basis of the severity of disease [35] and the patient’s age [17]. An efficient diagnosis approach [14] and early therapy initiation are common characteristics in various studies [12, 14, 33, 35, 36].
5. Conclusion
We showed TPE to have a high tolerance and strong safety profile in various ANDs. Furthermore, the improvement in mRS scores reflects the effectiveness of TPE in the treatment of MG, GBS, NMOSD, and AE
![]()
Therapeutic Plasma Exchange in Adults with Severe COVID-19 Infection
Objectives To evaluate the therapeutic use of plasma exchange in COVID-19 patients compared to controls. Methods Case series of critically ill adult men and non-pregnant women, ≥18 years of age, with laboratory confirmed COVID-19, was conducted at the Royal Hospital, Oman, from April 17th to May 11th, 2020. Therapeutic plasma exchange (TPE) was performed on patients admitted to intensive care unit (ICU) with confirmed or imminent acute respiratory distress syndrome (ARDS) or severe pneumonia. Analysis was performed using univariate statistics. Results A total of 31 COVID-19 patients were included with an overall mean age of 51 ± 15 years (range: 27-76 years), 90% (n = 28) were males, and 35% (n = 11) of the patients had TPE as a mode of treatment. The TPE group was associated with higher extubation rates than the non-TPE cohort (73% versus 20%; p = 0.018). Additionally, patients on TPE had a lower 14 days (0 versus 35%; p = 0.033) and 28 days (0 versus 35%; p = 0.033) all-cause mortality compared to patients not on TPE. However, all-cause mortality was only marginally lower in the TPE group compared to the non-TPE group (9.1% versus 45%; p = 0.055; power = 66%). Laboratory and ventilatory parameters also improved with the TPE. Conclusions The use of TPE in severe COVID-19 patients has been associated with improved outcomes, however, randomized controlled clinical trials are warranted to draw final conclusive findings
![]()
Case Report: Therapeutic and immunomodulatory effects of plasmapheresis in long-haul COVID
https://f1000research.com/articles/10-1189
Many patients with COVID-19 experience a range of debilitating symptoms months after being infected, a syndrome termed long-haul COVID. A 68-year-old male presented with lung opacity, fatigue, physical and cognitive weaknesses, loss of smell and lymphocytopenia. After rounds of therapeutic plasma exchange (TPE), the patient returned to normal activities and work. Mechanistically in the patient’s peripheral blood mononuclear cells (PBMCs), markers of inflammatory macrophages diminished and markers of lymphocytes, including natural killer (NK) cells and cytotoxic CD8 T-cells, increased. Circulating inflammatory proteins diminished, while positive regulators of tissue repair increased. This case study suggests that TPE has the capacity to treat long-haul COVID.
Keywords
Immunomodulation, Long Haul Covid19, plasmapheresis, adaptive immunity, inflammation, proteomics, leukocyute subsets
Corresponding authors: Dobri D. Kiprov, Irina M. Conboy
Competing interests: Dobri Kiprov and Regina Rohe are owners of Global Apheresis, which performs the procedure used in this case study. 655 Redwood Highway, Suite 370, Mill Valley, CA 94941
Grant information: This research was supported by NIBIB (R01 EB023776), NIA (1R01AG071787 and R56 AG058819), NHLBI (R01 HL139605), Open Philanthropy, Sillicon Valley Community Foundation, Foster Foundation, San Francisco Foundation, Georges’ Harik Foundation, Donors’ Trust grants to IC.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright: © 2021 Kiprov DD et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
How to cite: Kiprov DD, Herskowitz A, Kim D et al.
Case Report: Therapeutic and immunomodulatory effects of plasmapheresis in long-haul COVID [version 1; peer review: awaiting peer review]. F1000Research 2021, 10:1189 (https://doi.org/10.12688/f1000research.74534.1)
Introduction
The symptoms of “long-haul” coronavirus disease 2019 (long COVID) are debilitating and prevent patients from working, which is projected to negatively impact the healthcare system and economic recovery.1 The most common symptoms of long COVID are dyspnea with abnormal chest radiograph (CXR) findings, extreme fatigue, cognitive impairment (described as “brain fog”), myalgias, anosmia, ageusia, headache and sleep disorder.2 Long COVID resembles myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) which is driven by autoantibodies.3,4 Therapeutic plasma exchange (TPE) was successfully tried in patients with severe COVID-195 and is typically used on patients with ME/CSF and other autoimmune disorders.6,7 Moreover, our recently published studies suggest that TPE re-sets the circulatory proteome to healthier states, attenuating the so-called cytokine storm and enhancing the systemic determinants of tissue repair.8,9 The encouraging results of these reports convinced us to try TPE on a patient with severe long COVID.
Case
A 68-year-old caucasian male attorney, who reported having been very physically active and capable of multitasking in a demanding executive position at work began to feel fatigued and short of breath on December 14, 2020. He visited an emergency room on December 18, 2020. His oxygen saturation was 90–93% on room air and he was sent home. His breathing and fatigue deteriorated, so he was admitted to the hospital on December 21, 2020. He tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by PCR and was treated with remdesivir and Decadron (dexamethasone). His status continued to worsen, and he was placed on high-flow oxygen. He was never intubated. After 11 days in the hospital, he was sent home on portable oxygen which he used intermittently.
Over the following four weeks, he felt extremely fatigued. He could only walk to the bathroom and back to bed. He could not focus on anything cognitively, which he described as “brain fog”. He was unable to do any work and could not even answer his emails. He reported he had no sense of smell nor taste. Sedimentation rate, CRP, D-dimer and ferritin were abnormal in clinical lab tests (Figure 1B). At that time, chest radiographs and a chest CT scan revealed ground-glass appearance areas diffusely spread throughout his lungs (Figure 1C). A PCR test for SARS-CoV-2 was negative. A serologic test for anti-SARS-COV-2 IgG antibodies was positive.
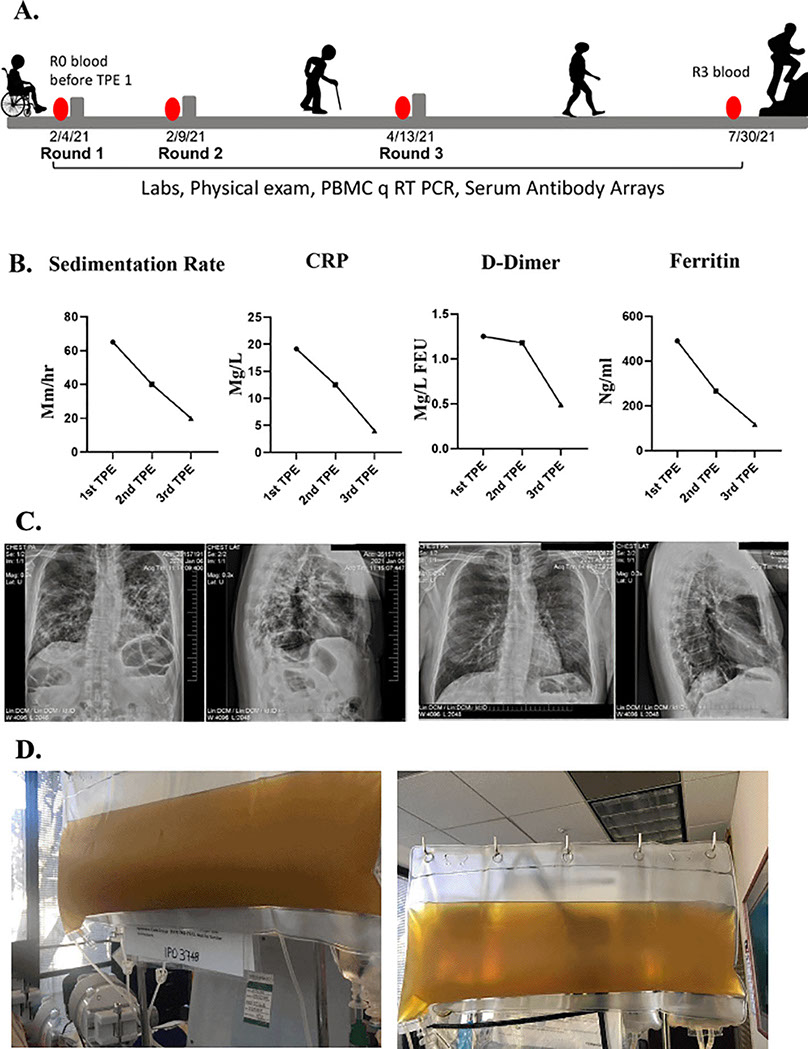
Figure 1. Clinical improvements in a long COVID patient.
A. Schematics of the case. B. Erythrocyte sedimentation rate, CRP, D-dimer, ferritin levels were assayed before each TPE procedure and were initially elevated but normalized by the TPE. C. Chest radiographs show reduced lung opacity after TPE. D. Clouded plasma appearance was reduced by TPE.
In February of 2021, he was seen in our clinic. At that time, he was very weak and unable to walk. When he arrived at the airport, he needed a wheelchair to go from the plane to the taxi. He underwent his first TPE on February 4, 2021. One plasma volume was exchanged, using 5% albumin as an exchange fluid. The removed plasma was very dark and opaque (Figure 1D). During his first TPE treatment, he was coughing profusely. After his first treatment, he could breathe more easily, and the cough subsided. The morning after the first TPE treatment, he could walk and was not struggling for breath. Two days later, he had no difficulty breathing and was able to walk 100 feet on a level surface but still had difficulty walking uphill.
He underwent a second TPE and two days after the second treatment he was able to walk uphill with ease and even jog. His brain fog disappeared, and he was able to get back to his daily work activities. Typical biomarkers of systemic inflammation all became quickly and robustly normalized including erythrocyte sedimentation rate, CRP, ferritin, and D-dimer10 (Figure 1B). The plasma from the second TPE was clear (Figure 1D). A week later his chest radiographs showed considerable clearing of the opacities in the lungs (Figure 1C). He also reported regaining his sense of smell and taste. He was seen in the clinic for another TPE two months later and, at that time, the patient reported that he was back to work, feeling like his normal self, and able to exercise daily without shortness of breath.
The peripheral blood mononuclear cells (PBMC) and blood serum of this patient were collected and analyzed before the first round of TPE (R0) and before each subsequent round (R1–R3) (Figure 1A). Real-time qRT PCR was used to study the levels of the markers of T cells: CD3; helper T-cells: CD4; cytotoxic T-cells: CD8; NK cells: CD94; B-cells: B220 and CD19; macrophages: CD11b; inflammatory myeloid cells: CD68; and IL-2 receptor: CD25. Before TPE, there was a prevalence of CD68+ inflammatory myeloid cells’ marker, and relatively fewer lymphocyte markers, which is indicative of a lack of adaptive immunity (Figure 2). TPE increased the markers of T-cells, B-cells, NK cells, and diminished the markers of inflammatory macrophages, suggesting enhanced adaptive immunity and attenuated inflammatory response (Figure 2). CD3, CD4 and CD19 initially diminished at 5 days after the first TPE, R1, but then steadily increased during the longer intervals of R2 and R3; CD94 and CD8, the markers of cytotoxic immune cells that combat viral infections also gradually and steadily increased (Figure 2).
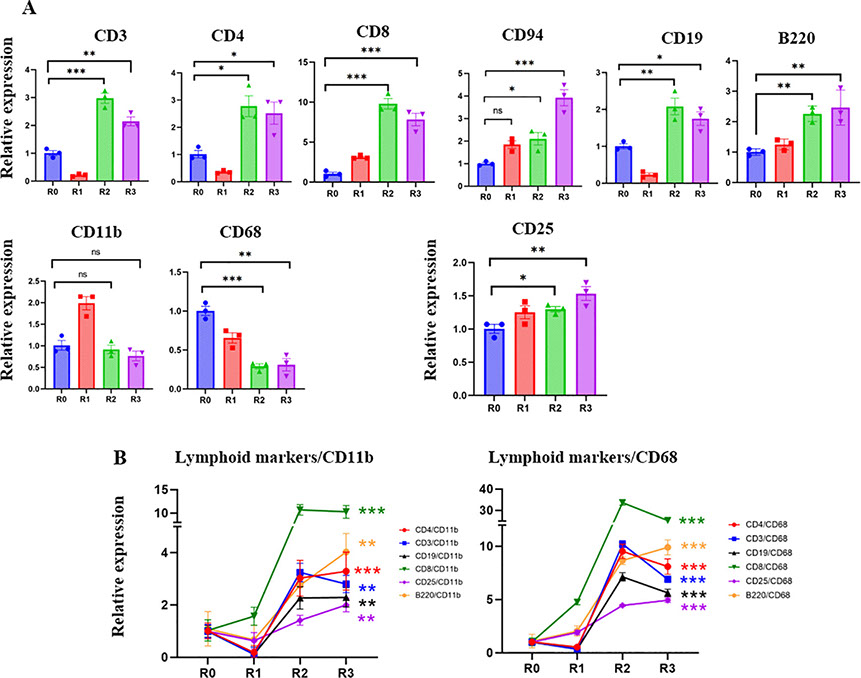
Figure 2. Effect of TPE on lymphoid and myeloid gene expression.
A. The expression of T cell, B-cell NK cell markers and IL2 receptor are significantly increased in R2 and R3 compared to R0. CD11b myeloid marker increased in R1 and then returned to R0 levels. CD68 – the marker of inflammatory myeloid cells, was significantly decreased by the third round of TPE. B. The ratios of lymphoid/CD11b, lymphoid/CD68, CD25/CD11b and CD25/CD68 markers clearly demonstrate the positive effects of TPE in promoting the adaptive rather than the inflammatory immune response. Note the break in Y-axis scale. *P < 0.05, P < 0.01, *P < 0.001.
Five days after the first TPE and the initial increase of CD11b+, the levels of this marker stabilized, while the CD68 marker of inflammatory macrophages were never elevated by TPE and were diminished by R2 and R3 (Figure 2). Plotting the ratios of T-cell and B-cell markers to myeloid and inflammatory macrophage markers (CD11b and CD68) demonstrates a rapid and robust shift toward adaptive immunity through the rounds of TPE (Figure 2). The effects of TPE are particularly striking when looking at the relative increase in the CD8+/CD68+, e.g., anti-viral cytotoxic T cells in an inflammation reduced environment (Figure 2).
Blood serum from the patient was profiled through comparative proteomics using RayBiotech antibody arrays, as we have published.8 Heatmapping clearly demonstrated the profound influence of TPE on the circulatory proteome (Figure 3A); Venn diagrams, KEGG and biological process databases uncovered 36 proteins that were commonly downregulated between the rounds of TPE, (Figure 3B–D). In agreement with the diminished inflammation and enhanced adaptive immunity that were suggested by Figures 1 and 2, this longitudinal serum proteomics revealed significant attenuation of inflammatory factors, which was stable and lasted several months (Figure 3E and F). Regulators of several growth factor pathways that are known to promote tissue repair increased in the systemic milieu: IGF, EGF, TGF-β (Figure 3E and F).
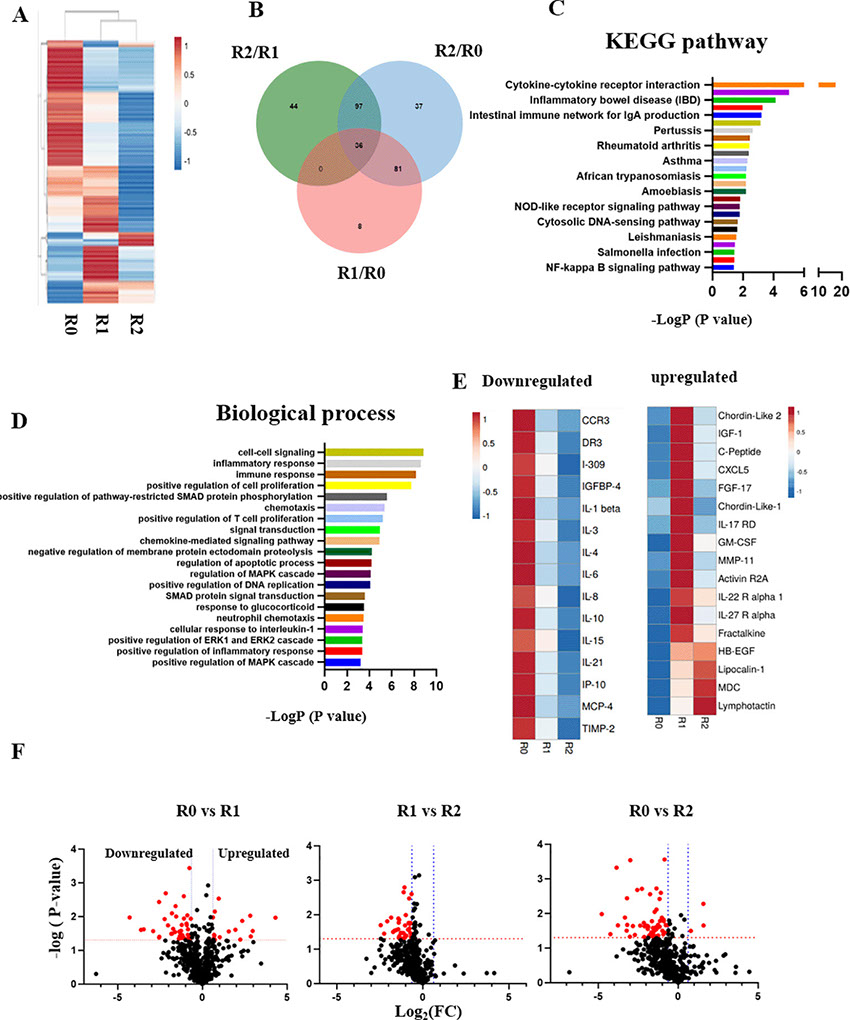
Figure 3. The longitudinal profiling of serum proteome after rounds of TPE.
A. Comparative heat mapping of 507 proteins between TPE rounds was done on RayBiotech Array. B. Venn diagram showing the overlap between the proteins, which were influenced by the TPE rounds. 36 shared proteins were diminished each TPE round (0.65> Fold change). C. The KEGG signaling pathway analysis. D. The top 20 list of biological processes. E. Heat mapping of the proteins that relate to Aging and Inflammaging and are decreased by TPE. F. Volcano plots of proteomics as per TPE rounds. More proteins are downregulated by the second round than by the first. The red dots represent differently expressed proteins (P < 0.05; |Log2FC|<1.5), while the grey dots represent proteins with P > 0.05.
These results establish rapid and significant changes in circulatory proteome of the patient, which are indicative of attenuated inflammation, productive immune response, and enhanced tissue maintenance.
Since the COVID-19 pandemic, roughly 70–80% of patients suffer various sequelae.11 About 40% of these patients have acute respiratory distress syndrome (ARDS) and one of the main sequelae is pulmonary fibrosis.12 Although the mechanism of COVID-19-induced ARDS may be different from classical ARDS, the onset and development of pulmonary fibrosis are commonly related to elevated inflammatory cytokines, such as IL-6.13 Accordingly, anti-inflammatory drugs attenuate COVID-19-induced pneumonia.14,15
In this study, a patient who was initially healthy, quickly developed health problems after being infected with the novel coronavirus. Moreover, after two months, his health deteriorated to the point of being unable to walk, being physically short of breath, suffering mental fatigue, and diffuse ground-glass appearance was observed throughout his lungs. Clinical and biological analyses strongly suggested systemic inflammation and a lack of productive immune response. After the first TPE treatment, the dyspnea disappeared, and cough subsided. The patient was also able to walk short distances. Interestingly, these changes were observed on day 2 after the first TPE treatment. A month later, the patient was able to walk uphill easily and jog. The decrease in concentration and cognitive fog disappeared, and the patient was able to return to normal life. During the same period, inflammatory macrophage markers robustly diminished and the markers of lymphocytes increased in the blood, suggesting that TPE promoted adaptive immunity and decreased inflammatory response. Such a productive immune response shift became prominent between the second and third rounds of TPE and rounds 1 and 2 were separated by only 5 days, e.g., too quickly for changing leukocyte subset numbers. Longitudinal comparative proteomics demonstrated that many proteins that are associated with inflammaging16 were attenuated by the rounds of TPE. For instance, pro-inflammatory factors, such as IL-1β, IL-6, and IP-10, stably decreased. In contrast, lipocalin-1 that participates in the sense of taste, and regulators of EGF, IGF, and TGF-β signaling pathways increased, demonstrating a better capacity for tissue maintenance and repair. Of note, the smell/taste reception returned to normal in this patient after TPE. In summary, this case study suggests that TPE may alleviate post COVID-19 sequelae via positive shifts toward adaptive immunity and tissue repair that are concurrent with reduction of inflammation
Removal of antibodies from red cells: Comparison of three elution methods https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3613657
Direct antiglobulin test (DAT) is the most common test done in immunohematology lab, which detects immunoglobulin and fragments of complement attached to the red blood cells. These coated red blood cells are difficult to accurately phenotype, which may be required for selection of appropriate unit of red blood cells for transfusion.
Aims:
We have studied the efficacy of various elution methods in removing the antibodies coating the red cells and their impact on different blood group antigen activity.
Materials and Methods:
Patient samples sent for serological evaluation of autoimmune hemolysis were included in the study. DAT and Indirect antiglobulin test (IAT) were performed using gel cards (ID system, DiaMed Switzerland). Antibody coated red cells, either by in-vivo or in-vitro sensitization, were used to assess the outcome of three elution methods.
Results:
Out of 93 DAT positive samples already sensitized in vivo, 28 (30 %) samples became DAT negative post elution using either of three methods, while 36 (38.8%) showed reduction in strength of reaction, whereas in 29 (31.2%) there was no change in strength of reaction. Similarly, out of the 17 samples prepared by in vitro sensitization, 12 samples became completely negative after glycine-HCl/EDTA elution, 9 and 5 samples became negative after heat elution and chloroquine diphosphate elution methods, respectively.
Conclusion:
On comparative analysis glycine-HCl/EDTA elution method was better than the other two methods and can be used for eluting immunoglobulins from intact red cells
![]()
'Young blood' and 'own blood' therapy for CFS https://forums.phoenixrising.me/threads/young-blood-and-own-blood-therapy-for-cfs.62605
Therapies with young (teens) people's blood and even patients own blood shows encouraging results for treating Alzheimer's and reversing some effects of aging. Studies being done in Stanford and elsewhere:
I am wondering if this could also be an option for CFS. CFS is rare among teens and when they get it, their chance of recovering is much higher than for adults.
Since CFS is a terrible disease, I think it would be ethically OK to try it, because it would be rather similar to giving a blood transfusion to a severely injured person. Also donating blood doesn't harm young people when done and supervised properly and it would be done on a voluntary basis.
Own blood therapy also seems to have some positive effects according to the article. No ethical problems would exist here.
Unable to work since July 2015, mostly bedridden since mid-2016.
Results of my physical exams (according to my physicians): https://www.youtube.com/watch?v=StTqXEQ2l-Y&feature=youtu.be&t=5
Please take nothing I am writing here as a recommendation to do something or not to do something. I just want to share information and experiences.
user Seven7 "I have heard good things is this what they called blood doping? What is funny is the
Sports people do all kind of crazy things and is “allowed” or sports turn the heads but for a bunch of sick propel which really need it for functioning is unheard off. We need to figure how this sports people do this enehancenents so we can do it!!!"
CFS/ME Videos https://www.youtube.com/channel/UCGuQZPHK85FADUlnLiR
user valentinelynx "I'm not yet impressed with the study of giving blood plasma to Alzheimer's patients. It was a small study (18 patients) funded by a biotech company. This will need much more study. Funny that the article you linked to misspelled the lead researcher (a neurologist)'s name: it's Sharon Sha, not Shaw. Here's a more complete description of the trial (it is intriguing): Clinical trial finds blood-plasma infusions for Alzheimer’s safe, promising
As for blood doping, it's similar: it involves either blood transfusions (not plasma) to increase hemoglobin levels, or injections of erythropoietin (to increase RBC production) or injection of synthetic oxygen carriers. The point of all of these is to increase the oxygen carrying capacity of the blood. And they are most definitely NOT allowed in sports: this is considered cheating, and will disqualify you from any official competition.
How do they do it? They pay some shady characters for it! If you really wanted to, and had the funds, I'm sure you could find a source. I'm not recommending it."
user Wonkmonk "I think as an experimental treatment, it would definitely not be illegal. It's not like we try to win the Olympics :rolleyes:
Unable to work since July 2015, mostly bedridden since mid-2016.
Results of my physical exams (according to my physicians): https://www.youtube.com/watch?v=StTqXEQ2l-Y&feature=youtu.be&t=5
Please take nothing I am writing here as a recommendation to do something or not to do something. I just want to share information and experiences."
user Gemini "Wonkmonk said: I am wondering if this could also be an option for CFS. Nancy Klimas used EPO a while back, I believe.
user Cort posted in 2016:
https://www.healthrising.org/forums/threads/more-oxygen-by-epo-how-in-natural-way.3511
user Wonkmonk "No one tried 'young blood' so far, right?
user Gemini "In his book "Faces of CFS" Dr. David Bell describes the case of a woman who had standard blood transfusions, improved, then relapsed. Treatment was not curative. Unknown if her transfusions were "young blood."
In 2015 user Cort and Invest in ME Research posted Dr. Bell's discussion of the case:
With Ron Davis' finding of "something in patients' blood," blood is an area of interest for sure.
Unknown if her transfusions were "young blood."
They certainly included a fraction of young blood, but perhaps only a very low fraction from teens.
Unable to work since July 2015, mostly bedridden since mid-2016.
Results of my physical exams (according to my physicians): https://www.youtube.com/watch?v=StTqXEQ2l-Y&feature=youtu.be&t=5
Please take nothing I am writing here as a recommendation to do something or not to do something. I just want to share information and experiences.
user knackers323 @Abdallah says he got benefit from having blood taken in this thread.
I wonder how it ties in with the finding that there is something in cfs patients blood
https://forums.phoenixrising.me/index.php?threads/hi-from-jordan.62107/page-2#post-1018735
Autoimmune disease: Could modified red blood cells lead to new treatments https://www.medicalnewstoday.com/articles/316227
New treatments that prevent and alleviate autoimmune diseases may come from using red blood cells to carry disease-specific proteins that retrain the immune system. So concludes a study that shows such an approach works in mice with multiple sclerosis and type 1 diabetes.
The researchers say that their method of using red blood cells to carry antigenic peptides could be an effective way to induce immune tolerance and alleviate symptoms of autoimmune diseases.
The study – led by the Whitehead Institute for Biomedical Research in Cambridge, MA – is about to be published in PNAS.
An autoimmune disease is one in which the immune system mistakenly attacks the body’s own cells, tissues, and organs.
There are more than 80 autoimmune diseases, and the more common ones include type 1 diabetes, multiple sclerosis (MS), systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease.
While the exact causes of many autoimmune diseases remain largely unknown, experts tend to agree that genes – together with infections and other environmental factors – play an important role.
A recent review of published evidence finds that global rates of autoimmune diseases have increased considerably over the past 30 years, particularly in industrialized nations.
Estimates from the National Institutes of Health (NIH) suggest that there are more than 23 million people in the United States living with autoimmune diseases.
No cures have yet been discovered for autoimmune diseases, although treatments that manage or alleviate the symptoms are available for many of them.
Applying the principle of ‘tolerance induction’
Many treatments involve giving patients drugs that suppress the immune system so that it does not overreact. However, such a blanket approach means that the immune system is not strong enough to tackle other illnesses.
Fast facts about MS
MS affects more than 2.3 million people worldwide
It affects two to three times as many women as men
The most common symptoms include extreme fatigue, mobility difficulties, visual disturbance, and altered sensation.
Learn more about MS
Researchers are therefore looking for ways to intervene only in that part of the immune system that is mistakenly reacting in the particular disease.
For the new study, the team sought to improve a technique called “tolerance induction,” which sounds good in theory but is proving difficult to apply.
The principle of tolerance induction is to use antigenic peptides or protein fragments from the particular cells that the immune system is overreacting to and retrain the immune system to ignore them.
However, scientists are finding the idea difficult to put into practice. For example, a particular stumbling block is getting the antigenic peptides to reach their destination before immune cells break them down.
To work around this, the team behind the new study decided to try a method that uses red blood cells to carry the antigenic peptides.
The team says that red blood cells are particularly well-suited to carrying the disease-specific antigen because they can reach almost every part of the body; they bring life-giving oxygen to cells.
Another reason is that red blood cells are recycled frequently – every month in mice and every 4 months in humans – without eliciting an immune system response.
Mechanism not well understood
For the new study, the researchers built on previous work in which they had attached a chemical tag and antibodies to red blood cells using a method that they called “sortagging.”
They used sortagging to attach antigens that trigger the disease-specific immune response to red blood cells from mice with MS and type 1 diabetes, and then transfused them back into the mice. The whole procedure took around 1 hour.
The results showed that the mice had reduced symptoms of the disease and even one transfusion before the disease developed was sufficient to lessen symptoms, note the researchers.
“Essentially what we’re doing,” explains first author Novalia Pishesha, a graduate student who carried out some of the work at the Whitehead Institute, “is hijacking the red blood cell clearance pathway, such that the foreign antigen masquerades as the red blood cells’ own, such that these antigens are being tolerated in the process.”
However, the researchers caution that while their results suggest that using red blood cells to carry antigenic peptides appears to be an effective way to induce immune tolerance, the underlying molecular and cellular mechanisms are not clear.
They suggest that their study offers a good starting point for further research into how the immune system regulates itself and why it sometimes does the wrong thing.
Nevertheless, co-senior author Harvey Lodish, a Whitehead founding member and professor of biology, says that: “This is a very promising step in the development of therapies for autoimmune diseases,” and concludes:
“If this type of response is also true in humans, then it could make a lot of these therapies possible for these diseases and similar conditions.”
Learn how stem cell transplantation may halt disease progression in MS.
![]()
Diabetes Multiple Sclerosis Blood / Hematology Immune System / Vaccines
Written by Catharine Paddock, Ph.D. on March 7, 2017
What to know about thrombocytopenia in children
Causes and types
Symptoms
Diagnosis and cause
Treatment
Summary
Thrombocytopenia refers to a low blood platelet count. This can lead to bruising easily or bleeding excessively.
In children, acute immune thrombocytopenia (ITP) is relatively common, and it may develop after a viral illness, such as chickenpox. In other cases, the condition is long lasting. It may stem from the use of medications or a genetic mutation.
If a child has acute ITP, they usually recover within 6 months, and if the condition is mild, they may not need any treatment.
Below, learn more about thrombocytopenia in children.
Causes and types of thrombocytopenia in children
Tony Anderson/Getty Images
A low blood platelet count can result from many factors.
Acute ITP
Researchers believe that viruses, such as the one that causes chickenpox, can temporarily alter how a child’s immune system behaves and cause it to attack platelets, resulting in acute ITP.
This issue is more common in children 2–6 years old. Usually, the symptoms are mild and go away on their own within 6 months. However, 1 in 5 children with the condition go on to develop chronic ITP.
Chronic ITP
This is rare in young children and is more common in adolescents and adults, particularly in females. It may last for several months or a lifetime and require specialist treatment. In some people, chronic ITP seems to resolve but recurs often.
Drug-induced thrombocytopenia
Thrombocytopenia can develop in response to medication, and this can be life threatening in newborns and other young children.
In a 2019 case study and review, a baby had a severe drop in their platelet count after receiving ranitidine (Zantac) for trouble feeding. Ranitidine can also treat acid reflux.
Fully understanding the effects of ranitidine and similar drugs on the platelet count in babies and young children requires more research.
Infections
Several infectious diseases can reduce platelet levels and cause secondary thrombocytopenia. Some of these illnesses include HIV, dengue fever, and malaria.
Malaria, in particular, can lead to a severe reduction in a child’s platelet levels, a 2021 study confirms. The authors suggest that the presence of thrombocytopenia may be a reliable indicator of malaria in children.
MYH9-related thrombocytopenia
This form of thrombocytopenia is distinct because the reduced platelet count results from a genetic mutation, rather than the immune system attacking the body’s platelets.
The MYH9 gene is responsible for building proteins that occur in certain blood cells, including platelets. A person can inherit this mutation from their parents or develop it spontaneously.
Several diseases involve a mutation of the MYH9 gene, and all of them can cause thrombocytopenia:
Epstein syndrome
Fechtner syndrome
May-Hegglin anomaly
Sebastian syndrome
Aplastic anemia
Aplastic anemia is a form of bone marrow failure, and it can cause thrombocytopenia. As the bone marrow shuts down, it produces fewer red and white blood cells and platelets.
In most cases, aplastic anemia develops without any obvious cause, and doctors refer to this as “idiopathic” aplastic anemia. However, some children may inherit the condition or develop it after an infection.
Bone marrow failure tends to occur between the ages of 1 and 5 years or between the ages of 12 and 20 years.
Symptoms of thrombocytopenia in children
One of the most telling symptoms is patches of bruising or purple areas of skin.
Additional symptoms include:
tiny red, rash-like spots
bleeding freely
bruising easily
having blood blisters in mouth
having blood in urine
heavy menstrual bleeding, in adolescents
Diagnosing thrombocytopenia and its cause
The National Heart, Lung, and Blood Institute explains that doctors consider a person’s medical history and the results of a physical examination and tests when diagnosing thrombocytopenia and identifying its cause.
Medical history
A doctor may ask about anything that could affect platelet levels, including:
the use of prescription and over-the-counter medicines
general eating habits
any family history of a low platelet count or related conditions
Physical examination
During this, the doctor checks for signs of bruising and bleeding. They may also look for symptoms of an infection, such as a fever.
Diagnostic tests
If the doctor suspects thrombocytopenia, they run several tests, which may also determine the cause of this issue.
These tests may include:
A complete blood count: The results can confirm low platelet levels.
A blood smear: This shows whether the platelets appear healthy.
Bone marrow tests: This confirms bone marrow health.
Prothrombin time test: This measures the blood clotting rate in seconds.
MEDICAL NEWS TODAY NEWSLETTER
Knowledge is power. Get our free daily newsletter.
Dig deeper into the health topics you care about most. Subscribe to our facts-first newsletter today.
Enter your email
Your privacy is important to us
Treatments
When a child has thrombocytopenia, the treatment depends on the cause and the severity of the symptoms.
For example, a child with mild acute ITP likely does not need treatment, as this tends to resolve on its own. However, if the child is highly likely to bleed profusely, they may need a blood or platelet transfusion.
A child with chronic ITP that frequently recurs may need medication that suppresses the immune system, such as rituximab. If an infectious illness, such as HIV, is responsible, the doctor may prescribe corticosteroids, which slow platelet destruction.
Overall, the goal of thrombocytopenia treatment is to prevent serious complications. If an underlying health condition is causing the low platelet count, the doctor will focus on treating both issues.
Summary
Thrombocytopenia refers to low levels of platelets in the blood, which can increase the risk of bleeding and bruising easily.
Acute ITP is the most common form of thrombocytopenia in children, and it usually resolves on its own within 6 months. Less common causes of thrombocytopenia include medications, infections, and a genetic mutation.
If a child’s symptoms are very mild, they may not need treatment. A child with chronic ITP may have a risk of severe bleeding and need immunosuppressant medication
Effective Blood Plasma Removal and Treatment https://utswmed.org/conditions-treatments/apheresis/therapeutic-plasma-exchange
Therapeutic plasma exchange (TPE), also known as plasmapheresis, removes and replaces a patient's blood plasma, The plasma portion of the blood is removed and replaced by a plasma substitute and then added back to the cells (red cells, white cells, and platelets) and finally returned by intravenous or central venous catheter access. The removed plasma is discarded.
procedure typically removes 65% to 70% of the disease-causing proteins (antibodies) in the plasma. Typically, several procedures are needed to lead to clinical improvement
Early and sustained improvement in fatigue-related quality of life following red blood cell transfusion in outpatients Outpatients with hematologic disease often receive red cell transfusion to treat anemia and fatigue. The effect of transfusion on fatigue-related quality of life and how well this effect is sustained has not been quantified. The study aim was to describe the early and sustained impact over four weeks of red cells on patient-reported fatigue in outpatients age ≥ 50 receiving transfusion as routine clinical care.
METHODS
FACIT-Fatigue scale scores were measured pre-transfusion and at visits targeting 3, 7, and 28 days post-transfusion. Group-based trajectory modeling of patient fatigue scores by study day was used to identify the number of distinct trajectories (Groups), then longitudinal mixed-effects modeling of fatigue scores was used to estimate group-specific mean improvements early after transfusion and between days 3 and 28 post-transfusion.
RESULTS
Four distinct fatigue score trajectory groups were identified and were found to be correlated with baseline fatigue scores (means 12, 26, 34, and 47 points). In the three groups with the lowest fatigue trajectories (indicating greater fatigue), improvements in fatigue early after transfusion achieved the established minimum clinically important difference (≥ 3 points, Group p=0.0039). In all trajectory groups, mean fatigue levels did not change significantly between 3 and 28 days (± 1 point, Group p=0.60).
CONCLUSION
Patient-reported fatigue varies widely among older adult outpatients with hematologic disorders. Nonetheless, trajectory modeling suggests that most anemic patients can expect a noticeable improvement in fatigue in the first few days after
page 21r CFS > ALLOPATHIC MEDICINES > BLOOD TRANSFUSION THERAPY
page 21c
page 22
This page is long and detailed as it's an exploratory (and complex) topic. You may skim or skip this page until it has been trialled for CFS. Click here to be notified of page or site updates

FBT may also address some of the Circulatory issues found with CFS patients (for details, please view 'Circulatory Impairment, Disease and Irregularities' section at the full report https://bra.in/7v2NY6)
- White Blood Cells/Leukocytes/Mast Cells/Immune Cells
- Abnormal Nocturnal Heart Rate Variability
- Abnormalities in Cerebral Perfusion
- Blood cells in chronic fatigue syndrome are drained of energy
- B-Lymphocyte Depletion
- Capacitance
- Cardiovascular Preload Issue
- Cerebral Blood Flow is Decreased in CFS
- Coagulation Defect
- Compensated Idiopathic Cardiomyopathy (A Model)
- Defective vasoconstriction producing POTS in CFS (with cardiac autonomic changes as a secondary response)
- Dysregulation of Protein Kinase Gene Expression in NK Cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Patients
- Endothelial Dysfunction
- Eripheral blood mononuclear cells (PBMCs)
- Glycolysis
- High White Blood Cell Count
- Hypercoagulation
- Hypoglycemea
- Hypoperfusion
- Hypotension
- Impaired ACh-Mediated Vasodilatation and ACh endothelial sensitivity/Dysfunction
- Intracranial Compliance (ICC)
- Lipotoxicity
- Low Blood Pressure
- Low Blood Volume (Hypovolemia
- Low NK Cell Activity
- NK Cells ERK1/2, MEK1/2 and p38 downstream signalling molecules impaired in CD56 dim CD16+ and CD56 bright CD16 dim
- Impaired calcium mobilization in natural killer cells (associated with transient receptor potential melastatin 3 ion channels)
- Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients
- Natural killer cells and single nucleotide polymorphisms of specific ion channels and receptor genes in myalgic encephalomyelitis/chronic fatigue syndrome
- Lower ambulatory blood pressure
- Lower Systolic Blood pressure
- Orthostatic intolerance
- Oxygen
- Impaired oxygen delivery to muscle
- Poor Oxygen Extraction is Contributing to Exercise Intolerance
- Metabolomics Study Suggests Chronic Fatigue Syndrome May Be Oxidative Stress/Low Oxygen Disease
- Low oxygen uptake by muscle cells causes exercise intolerance in a majority of CFS patients, indicating insufficient metabolic adaptation to incremental exercise
- Red blood cells in ME/CFS demonstrate reduced ability to change shape
- Poor Erythrocyte Deformability
- Prolonged acetylcholine-induced vasodilatation in the peripheral microcirculation of patients with chronic fatigue syndrome
- Reduced Absolute Cortical Blood Flow in CFS
- Reduced Blood Flow
- short QT interval
- Small Left Ventricle
- Stagnant Hypoxia
- Systemic Exertion Intolerance
![]()
Abnormalities in Cerebral Perfusion
From: A Comparison of Neuroimaging Abnormalities in Multiple Sclerosis, Major Depression and Chronic Fatigue Syndrome (Myalgic Encephalomyelitis): is There a Common Cause?
https://link.springer.com/article/10.1007/s12035-017-0598-z
Most of the SPECT studies investigating potential CBF abnormalities in CFS have reported areas of significant cerebral hypoperfusion, at either a global [20, 192] or regional level [193, 194] compared with unaffected controls. However, a minority of researchers reported no significant CBF impairments in comparison with unaffected controls or MDD participants [195, 196]. The reasons for such discrepant findings are unclear, but fewer participants and differences in selection methods may be relevant. The much larger studies conducted by Ichise, Schwartz, Costa and Goldstein, and their colleagues [20, 192,193,194] appear worthy of particular consideration as they examined multiple brain regions and perhaps even more importantly, apart from Ichise and others [192], examined differences in patterns of abnormal perfusion between participants with CFS and MDD, which is currently a contentious area.
Ischise et al. compared rCBF in 60 CFS participants and 14 healthy controls and reported significant reductions in cortical/cerebellar rCBF ratios in the frontal, temporal, parietal and occipital lobes, providing objective evidence of widespread functional impairment of the brain in the vast majority of their CFS subjects [192]. Schwartz et al. examined SPECT data differences in terms of regional perfusional deficits and mid-cerebral uptake indices between 45 CFS participants, 14 MDD participants, 27 participants with AIDS dementia complex (ADC) and 38 healthy controls [20]. These authors noted more areas of impaired frontal and temporal perfusion in the ADC group compared with those with CFS or MDD. However, the mid-cerebral uptake index was lower in patients with ADC and CFS than in the MDD and healthy controls groups [20].
The largest study examining potential 99mTc-HMPAO SPECT differences between CFS and MDD patients was carried out by Costa and others [193]. It included 40 healthy participants, 67 CFS participants (both with and without psychiatric co-morbidity) and 29 earlyand late-onset MDD participants [193]. These authors reported significantly greater global and brain-stem hypoperfusion in CFS participants free of psychiatric symptoms compared with controls and MDD participants, which was not apparent in CFS participants displaying symptoms of anxiety and depression [193]. Goldstein and others compared high-resolution 99mTc-HMPAO SPECT imaging patterns of rCBF in 33 CFS subjects (55 ± 10 years), 26 age-matched MDD participants and 19 healthy participants in an attempt to investigate the potential pathophysiology of CFS [194]. Importantly, they reported dorsofrontal hypoperfusion as the dominant profile in CFS while hypoperfusion seen in MDD was predominantly in the right orbitofrontal lobe, left temporal lobe and, in particular, the left anterior frontal lobes [194].
More recent research using xenon-computed tomography, ASL and high-resolution SPECT reported consistently impaired CBF at a global and regional level at rest and during exercise [197,198,199,200]. Yoshiuchi and others reported reduced CBF in the right and left hemisphere in CFS participants free of psychiatric comorbidity compared with healthy controls but also noted that hypoperfusion in CFS patients with psychiatric comorbidity appeared to be exclusively limited to the left hemisphere [198]. These results have been broadly replicated by Biswal and others who reported impaired regional and global CBF in the vast majority of their participants with evidence of regional hyperperfusion in a minority [197]. Evidence of hyperperfusion in some CFS subjects has also been supplied by Machale and others who reported significantly increased hyperperfusion in the left thalamus in CFS compared with a pattern of hyperperfusion in the left prefrontal cortex of MDD [200]. Finally, Patrick-Neary and others reported significantly decreased CBF in CFS participants during maximal exercise compared with healthy controls which they suggested as a cause of the profound disability experienced by many patients [199].
The potential capacity of SPECT to differentiate CFS from MDD may be highly significant as CFS is a purely descriptive diagnosis reliant on phenotype only, and there is accumulating evidence that the diagnosis identifies a clinically and aetiologically heterogeneous syndrome rather than a discrete biological entity even when such a diagnosis is made via the application of international guidelines [1, 3, 201, 202]. Unsurprisingly, research in this area has been bedevilled by a lack of reproducibility which is also the case in many other areas where diagnostic categories are descriptive and homogeneity of causation is assumed (reviewed in [203]). This issue in ‘CFS research’ is even more complex because of the frequent use of unvalidated criteria which only mandate the presence of chronic mental and physical tiredness [204] or where a diagnosis is based on the presence of intermittent symptoms based on a population study of fatigue in a localised population [205]. Hence, a method for increasing the homogeneity of a study population and excluding patients with MDD is to be welcomed. The question of heterogeneity is also relevant to the next section of this paper which briefly examines mechanisms whereby systemic inflammation could be a major element initiating and maintaining neuropathology in different illnesses even though investigation with an array of neuroimaging techniques produces very different and often illness-specific results
Factors Affecting Severity and Specificity of Neuropathology
There is widespread agreement that the relationship between the presence of systemic inflammation and various dimensions of neuropathology is established via the conveyance of inflammatory signals to the brain through a range of humoral and neural routes culminating in the activation of microglia and astrocytes [206, 207]. Once activated, these glial cells secrete a range of inflammatory molecules such as pro-inflammatory cytokines, reactive oxygen species, reactive nitrogen species, prostaglandins and quinolinic acid [208, 209]. These are well-documented effects and have been described in a number of detailed reviews [210, 211].
Another source of neuropathology is more indirect however and stems from the loss or corruption of the regulatory functions exerted by these glial cells via a sophisticated communication system with neurones and oligodendrocytes founded on the secretion of exosomes containing mRNAs, miRNAs, caspases and P2X7 (reviewed in [212]). This is a highly complex area, but the key point from the perspective of this paper is that abnormally expressed microglial exosomal miRNAs appear to be independent contributors to the development maintenance and illness-specific patterns of neuroinflammation seen in many different neurodegenerative and neuroprogressive disorders [213]. Thus, microglial activation following prolonged systemic inflammation could produce an illness-specific or at least a range of characteristic WM abnormalities depending on a particular signature of abnormally expressed miRNAs. The mechanisms underpinning this phenomenon are currently unknown but may lie in the recently discovered capacity of at least some miRNAs to activate toll-like receptors and are another mechanism underpinning ‘sterile’ immune activation [214, 215].
Microglia also display distinct regional differences in density and transcriptional identity particularly in bioenergetic and immunoregulatory pathways [216, 217]. This underpins observations of region-specific sensitivities to microglial dysfunction and in particular responses to different pathogen-associated molecular patterns [216, 217]. Hence, this provides another mechanism whereby systemic inflammation could produce geographically variable neuropathology. Another highly pertinent example of the role of microglial genetics in creating unique patterns of abnormalities can be found from recent data in MS, where microglia deficient in the production of caspase-8 are considered to be the source of necroptotic markers seen in active plaques of MS patients. These glial cells are likely one of the main drivers of neuronal and oligodendrocyte destruction seen in this illness [218, 219]. The heterogeneous responses of microglia to external or internal stimuli are also under epigenetic regulation involving DNA methylation, histone acetylation and the expression of a wide range of miRNAs [220]. It is also noteworthy that different genomic patterns of DNA methylation within and outside glial cells are associated with different patterns of neuropathology in distinct brain regions [221].
Predictably, astrocytes also display extensive variability in biochemical, biophysical and immunoregulatory properties in distinct brain regions (reviewed in [222, 223]). The signalling pathways orchestrated by astrocytes are also strongly influenced by genetics [224]. Importantly, some of the neuroprotective functions of astrocytes are also under epigenetic control. Reactive microglia also compromise astrocytic function and survival in an inflammatory environment with the latter property resulting from a decrease of histone acetylation in astrocytes and a silencing of Nrf-2-mediated antioxidant defences [225].
Antioxidant defence systems also demonstrate pronounced regional differences in composition and efficacy. For example, thioredoxin and thioredoxin reductase levels vary in different brain regions [226]. This is also true of glutathione transferase, glutathione reductase and glutathione peroxidase, with the former two enzymes being reduced in the striatum, neocortex, cerebellum and hippocampus, whereas expression of the last enzyme appears to be reduced in the striatum, cerebellum, cortex and corpus callosum [227, 228]. The expression and activity of NADPH oxidases also displays considerable variation in different brain regions [229]. Selenium distribution across different brain regions is also heterogeneous, with the highest concentrations being in the parietal inferior lobule, putamen and occipital cortex and the lowest levels in the cerebellum and medulla [230].
Different discrete neuronal populations existing in different brain regions display differential susceptibility to neurodegenerative stressors which is a phenomenon often described as selective neuronal vulnerability (SNV). Well-documented examples include neurones in the entorhinal cortex, frontal cortex, hippocampal CA1 region and amygdala, which are the most susceptible to neurodegenerative death in populations of neurones most sensitive to the neurodegeneration associated with Alzheimer’s disease [231, 232]. The increased susceptibility of dopaminergic neurones of the substantia nigra to neurodegenerative destruction in Parkinson’s disease is equally well documented [233, 234]. It should be stressed that the pattern and extent of neural damage seen in different neurodegenerative disease is not just dependent on SNV but also stems from specific factors unique to the aetiology of the illness (reviewed in [235]). SNV also exists within populations in the same brain region. For example, the hippocampal CA1 neurones are much more vulnerable than are CA3 neurones to global cerebral ischaemia [236, 237] and oxidative stress [238, 239].
Brain volume and structure are strongly influenced by genetic factors [240,241,242] and resting-state functional connectivity [243, 244], GM and WM volume [245, 246] and CBF [247, 248] are sensitive to environmentally induced epigenetic changes in gene methylation and/or acetylation and/or changes in the expression of a wide range of miRNAs. These factors may also produce heterogeneity in neuroimaging phenotypes between individuals with the same illness or between illnesses even if systemic inflammation is a major driver of neuropathology in each instance
![]()
CFS is associated with cerebral hypoperfusion, while large VBM studies have shown evidence of GM and WM changes
The evidence given in this paper points to the likelihood that systemic inflammation may be a major underlying cause of structural and functional neuroimaging abnormalities reported in seemingly diverse disorders including MS, MDD and CFS
Researchers looked specifically at the metabolic processes of oxidative phosphorylation https://en.wikipedia.org/wiki/Oxidative_phosphorylation and glycolysis https://en.wikipedia.org/wiki/Glycolysis two ways cells break apart chemical fuel to transfer energy in respiration.
White blood cells taken from 52 patients with CFS and 35 controls were put through their paces under optimal and stressful conditions, testing their capacity to deal with low oxygen levels.
There appeared to be a number of key differences in their metabolic processes. But none were as dramatic as the contrast in maximum levels of respiration.
By forcing the cells to boost their energy production, the researchers found those with CFS could only squeeze about another 50 percent from their cells – unlike the controls, who nearly doubled their output.
"The CFS cells couldn't produce as much energy as the control cells," says Tomas http://www.meassociation.org.uk/2017/11/new-scientist-blood-cells-in-chronic-fatigue-syndrome-are-drained-of-energy-04-november-2017
"At baseline, they didn't perform as well, but the maximum they could reach under any conditions was so much lower than the controls."
While the research focused on just one type of cell, it is an important step towards establishing a link between the symptoms of muscle pain, lethargy, and impeded cognitive functions and a biochemical process
For more on consistent abnormalities found in CFS patients visit https://bra.in/8pK4xB
See also UBI, IVIG, Ozone,
![]()
Extracorporeal Apheresis
Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? - PMC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8209771
"extracorporally filtered and cleaned with the use of Heparin, which is then removed again before the blood is being fed back into the body. The Heparin binds to various inflammation inducing components in the blood plasma"
Apheresis

back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top
back to top












