Probiotic Supplements Review (Including Pet
Probiotics)
Find the Best Probiotic
Supplement. CL Tests Reveal the Best Probiotic Supplements for You (and your
pet)!
Medically reviewed and
edited by Tod Cooperman, M.D. 
Last Updated![]() : 12/06/2021 | Initially Posted:
04/05/2020Latest Update: Probiotic for Colds?
: 12/06/2021 | Initially Posted:
04/05/2020Latest Update: Probiotic for Colds?

Recent Reviews
·
Aloe Juices, Gels, and Supplements
Review
·
NAD Booster Supplements Review
(NAD+/NADH, Nicotinamide Riboside, and NMN)
·
PQQ (Pyrroloquinoline Quinone)
Supplements Review
Table of Contents
Summary
What are probiotics? Probiotics are viable (living or
hibernating) bacteria and/or yeasts that confer a health benefit. Probiotics
are sold as cultured foods and beverages, such as yogurts and kefirs, as well
as in capsules, tablets, and powders.
What are the health
benefits of probiotics? Certain probiotic strains may be helpful for constipation,
bloating, diarrhea and irritable bowel syndrome, while others may be helpful
for throat and respiratory infections, and other uses.
What are the best
probiotic supplements? We suggest that you check our Results table
to find identify products "Approved" for quality in our testing. Then
consider the following:
·
Choose a product that contains the type(s) and amounts of
probiotic organism(s) shown to work for your condition. See the "What They Do"
section and then check the Results table
for products that contain that/those organism(s).
·
See our Top Picks for
some of the most common uses of probiotics.
·
Be aware that there can be huge variation in the number of viable
cells (CFUs) from product to product. Among products tested, this ranged from
just 100 million to hundreds of billion!
Typically, an adult probiotic should provide at least 1 billion cells daily —
although, as discussed in the "What They Do"
section, some probiotics have been shown to work at a lower dose.
Caution: If you have a milk
allergy, be aware that trace amounts of milk proteins may occur in some
probiotics (see Concerns and
Cautions).
UPDATES: Mary
Ruth's Liquid Probiotic
(11/2/21) Mary Ruth Organics issued a recall of
two lots of its Liquid Probiotic for Infants due to the
potential for bacterial contamination with Pseudomonas aeruginosa,
the same organism that ConsumerLab reported finding in Mary Ruth's
Liquid Probiotic in 2020, which Mary Ruth's disputed, as described
below.
(4/23/20) CL was contacted this month by a representative of Mary
Ruth Organics for more details about our tests of its product, Mary
Ruth's Liquid Probiotic, which was Not Approved in this Review due to two
independent laboratories finding it to be contaminated with Pseudomonas
aeruginosa by DNA sequencing.
Mary Ruth Organics disputed CLs results, providing certificates of analysis
supplied by its manufacturer of the product from two different laboratories
that apparently tested product from the same batch as the product we tested
(lot # 210920190). The certificates each indicate the product was negative
for Pseudomonas aeruginosa. These laboratories claimed to use the
same USP methodology applied by the laboratories that tested the product for
CL. However, CL has no reason to believe that the results it received in its
testing are incorrect, particularly as all probiotics in this Review were
similarly tested and no other product was positive for Pseudomonas.
Per its protocol, ConsumerLab
retains an unopened sample of every product it reviews. CL has offered Mary
Ruth Organics the opportunity to have that sample tested at a third-party
laboratory of mutual acceptance so long as Mary Ruth Organics is willing to
publish the findings for the public on its website, which ConsumerLab would
also do. ConsumerLab would pay to ship the product overnight to the chosen
laboratory and Mary Ruth Organics would directly pay the laboratory for the test.
CL will update this post if Mary Ruth Organics accepts this retesting
opportunity and, again, when test results are obtained.
What They Are:
Probiotic
products consist of viable (live) bacteria and/or yeasts that confer a health
benefit. Probiotics are available in varied forms such as yogurt and other
cultured milk foods, capsules, tablets, beverages, and powders. Probiotics
should not be confused with prebiotics, which are complex sugars (such
as inulin and other fructo-oligosaccharides) that are ingested as fuel for
bacteria already present in the gastrointestinal tract. Prebiotics and
probiotics are sometimes combined in the same product and termed synbiotics.
Although not included in this current Review, in past years, ConsumerLab has
also tested probiotic-containing fermented drinks: kefir and kombucha.
Kefir
In 2015, ConsumerLab tested three popular kefir (cultured
milk) products (Evolve, Lifeway, and Latta) and
found huge amounts of live cells per cup (respectively, 950 billion, 250
billion, and 150 billion cells). Interestingly, although people with lactose
intolerance are often advised to consumer kefir instead of milk, all the kefirs
contained lactose, ranging from 8.2 to 12.7 grams per cup, nearly as much as in
milk. However, enzymes from the bacteria in kefir may help breakdown lactose in
the digestive tract.
A 2021 study of five kefir sold in the U.S. found that none
of the three products that guaranteed live cell counts on their labels
contained their promised amounts. The researchers wrote that Lifeway
Original Kefir promised 30 billion live cells per gram but only 1
billion was found, Redwood Hill Farm Plain Goat Milk Kefir claimed
100 billion cells but contained 22 million, and CoYo Kefir claimed
1 billion cells but contained only 189 million. Maple Hill Plain Kefir and Siggi's
Plain Filmjö did not make live cell count claims and were found to
contain, respectively, 203 million and 473 million live cells per gram. In
addition, not all of the products contained the probiotic species listed on
labels. For example, the presence of only three of the 11 probiotic strains
listed on Lifeway's label could be confirmed, including none of
its listed Bifidobacterium. Interestingly, the one yogurt the
researchers tested, Chobani Plain Yogurt 0% Milk Fat, met its label
claim of 10 billion cells per gram, and the researchers were able to confirm
the presence of 4 of the 5 probiotic species listed on its label (Metras, JDS Communications 2021). It
must be noted that an industry group representing some of these brands
issued a response in June
2021 countering some of these findings, including the contention from Lifeway that
its claim of 30 billion live cells was in reference to a one-cup serving,
not a single gram of kefir.
Kombucha
Kombucha is a probiotic beverage produced by fermenting sweetened black or
green tea with bacteria and yeast. Depending on the type of tea, sugar, and
starter bacteria and yeast used, the resulting liquid contains varying amounts
and types of bacteria and yeast, tea catechins (such as epigallocatechin
gallate i.e. EGCG), organic acids (including acetic, lactic and gluconic acid),
caffeine, sugars (sucrose, lactose, glucose, and fructose) and small amounts of
ethanol (alcohol), amino acids, vitamins and minerals (Greenwalt J Food Proct 2000).
As a result of fermentation, kombucha is naturally lightly carbonated with a
slightly vinegary taste. Fruit juices and/or spices and other ingredients may
be added for flavor or to provide the sugar that is fermented. (See ConsumerTips™).
The starter culture of yeast and bacteria used to ferment the tea is called a
SCOBY (symbiotic culture of bacteria and yeast), Medusomyces gisevi,
or "mother" (a term also used to describe the culture in apple cider vinegar).
Due to the mushroom-like shape and brown color of the SCOBY, pieces of which
may remain in the liquid after processing, kombucha is also referred to as
"mushroom tea."
(For the clinical evidence regarding kombucha see the end of the next section).
What They Do:
The normal human gastrointestinal tract contains hundreds of
different species of bacteria, referred to as intestinal flora. When the normal
balance of these bacteria is disturbed by illness or antibiotic treatment, the
most common effect is diarrhea. Probiotics were originally thought to work by
re-colonizing the small intestine and crowding out disease-causing bacteria,
thereby restoring balance to the intestinal flora. However, research is showing
that probiotics are more likely to act in other ways, such as producing
substances that inhibit disease-causing bacteria, competing for nutrients with
them, stimulating the body's own immune system and interacting with nervous
system present in the gut. For example, a U.S.
government-funded study with Lactobacillus GG (Culturelle),
showed that giving older, healthy individuals a capsule with 10 billion cells
twice a day for 28 days caused no significant change in the composition of the
intestinal flora but appeared to modulate bacterial activity in ways which
could promote interactions with the gut lining and anti-inflammatory pathways.
When retested a month after treatment ceased, the effects were no longer
present — indicating that the probiotic was only effective during and/or
shortly after administration (Eloe-Fadrosh, Mbio 2015). An analysis which looked at this and seven other studies of
probiotics concluded that there is "a lack of evidence" as to
"whether or not there is an effect of probiotics on the fecal microbiota
composition in healthy adults" (Kristensen, Genome Medicine, 2016).
It is important to note that this analysis (funded in-part by an unrestricted
grant from the controlling entity of the pharmaceutical company Novo Nordisk
A/S) assessed only the bacterial composition of feces and not the effects of
probiotics in the gut itself or in the treatment or prevention of disease,
i.e., it is not a commentary on the clinical effects of probiotics. A study that used endoscopy to take samples directly from within
the intestines found that, after taking a multi-strain probiotic (providing 25
billion cells) twice daily for two months, only 2 of the 10 participants had
significant colonization in the gut, and four had moderate colonization. The
researchers concluded that colonization does not occur in everyone who
takes probiotics and may depend on factors such genetic and immune
system differences (Zmora, Cell 2018).
A variety of probiotic organisms (alone or in combination) have been tested in
clinical trials for a range of conditions. Here are some of the most notable
findings by condition:
Symptoms of irritable bowel syndrome (IBS)
In adults:
As noted below, certain probiotics have been shown to improve symptoms of IBS
in adults, but the appropriate probiotic may depend on whether the IBS is
predominantly causing diarrhea or constipation. Be aware, however, that
the American Gastroenterological Association does not recommend
the use of probiotics in treating IBS, having found supporting evidence to be
insufficient (Grace, Gastroenterology 2020).
Bifidobacterium infantis 35624 (used in Procter &
Gamble's Align) was found to improve symptoms (e.g., bloating,
straining, gas) of irritable bowel syndrome (IBS) in women,
although it did not change the frequency of stools (Whorwell 2006). However, not all studies using
this strain have shown a benefit. In another study of this strain, funded by
Procter & Gamble, one capsule containing 10 billion cells of
freeze-dried B. infantis 35624 (Align) taken once daily
for three months did not improve symptoms of IBS compared to
placebo (Charbonneau, Gut Microbes 2013).
A multi-strain probiotic drink was found, in a 12-week study, to modestly
reduce IBS symptoms in adult women and men (Sisson Aliment Pharmacol Ther 2014).
Based on a 500-point symptom severity scale, there was a 63.3 point decline
among those taking the probiotic compared to a 28.3 point decrease among those
getting a placebo. The decline with the probiotic was largely due to decreases
in pain and improvement in bowel habit satisfaction. There was no significant
improvement in bloating or with overall quality of life. The probiotic (sold
as Symprove in England) is a combination
of Lactobacillus rhamnosus NCIMB30174, Lactobacillus
plantarum NCIMB 30173, Lactobacillus acidophilus NCIMB 30175,
and Enterococcus faecium NCIMB 30176 in a water-based
suspension of barley extract with 10 billion live organisms per 50 mL dose
which was kept refrigerated and taken each morning on an empty stomach 20
minutes before breakfast.
Capsules of a strain of Saccharomyces cerevisiae (commonly
known as Brewer's yeast) were shown, in an 8-week, placebo-controlled trial in
adults with IBS, to significantly improve abdominal pain/discomfort during the
last 4 weeks of treatment. Sixty-three percent of those receiving the probiotic
reported improvement versus 47% of the placebo group. There was also a trend of
improvement in other symptoms (bloating/distension, bowel movement difficulty),
but these were not statistically significant. There was no effect on stool
frequency and consistency. People in the study took a single capsule each day
with water at breakfast: one capsule contained 4 billion cells of Saccharomyces
cerevisiae CNCM I-3856 (Lynside Pro GI+, Lesaffre International
-- sold in France and in some U.S. products) (de Chambrun, Digestiv and Liver Dis
2014). A larger study in which adults with
IBS took two capsules daily of the same probiotic strain (providing a total of
8 billion cells of Saccharomyces cerevisiae CNCM I-3856) for 3
months also found abdominal pain and bloating were significantly reduced (by approximately
11% and 13%, respectively) compared to placebo, but only among those with constipation-predominant IBS.
Those with diarrhea-predominant or "mixed" IBS did
not have significant improvements compared those receiving placebo (Spiller, United European
Gastroenterol J 2015). Previous research with this strain
demonstrated an analgesic effect in the gut of animals 15 days after beginning
administration, with the effect disappearing 3 days after the last
administration (Rousseaux, Gastroenterology 2010).
An eight-strain combination of Lactobacillus,
Bifidobacterium and Streptococcus (Visbiome,
formerly sold as VSL#3 — see What to Consider When Buying for more
about this) reduced abdominal bloating in patients with diarrhea-predominant IBS
but had no effect on other symptoms such as abdominal pain, gas and urgency (Kim, Aliment Pharmacol Ther 2003).
Among people with diarrhea-predominant IBS,
a tablet containing 2 billion spores of B. coagulans MTCC 5856
(sold as LactoSpore from Sabinsa Corporation) decreased
bloating, vomiting, diarrhea, abdominal pain, and stool frequency compared to
placebo over 90 days in a small study in India. Self-reported disease severity
also decreased and quality of life increased. The improvements began during the
first month and generally increased during 90 days. The tablet was taken once
daily at least 30 minutes before a meal --typically breakfast, while patients
also received standard medical treatment. (Majeed, Nutr J 2016)
[Although no LactoSpore products were tested in this
Review, Digestive Advantage (Schiff) was tested and Approved
and provides 2 billion spores of another strain of B. coagulans per
capsule]. A study among 400 men and women in
Bangladesh with moderate-to-severe symptomatic diarrhea-predominant IBS
(IBS-D) found that those who took 2 capsules of a blend of 14 bacterial strains
(Bio-Kult by Protexin, which funded the study) twice daily before
or during a meal for 3 ½ months had, compared to placebo, slightly fewer bowel
movements per day (2.7 vs. 3.5) and a significant reduction in self-reported
symptoms (69% vs. 49% reduction in symptoms such as abdominal pain and
interference with quality of life). Each capsule of Bio-Kult (which is widely
available in the U.S.) contained 2 billion cells of the following
strains: Bacillus subtilis PXN 21, B. bifidum PXN
23, B. breve PXN 25, B. infantis PXN
27, B. longum PXN 30), Lactobacillus spp. (L.
acidophilus PXN 35, L. delbrueckii spp. Bulgaricus PXN39, L.
casei PXN 37, L. plantarum PXN 47, L.
rhamnosus PXN 54, L.helveticus PXN 45, L.
salivarius PXN 57), Lactococcus lactis PXN 63,
and Streptococcus thermophilus PXN 66 (Ishaque, BMC Gastroenterol 2018).
Some yogurts are now fortified with additional probiotic
strains and have been marketed as promoting "digestive health,"
although it is not clear if they can help with IBS. Bifidobacterium
lactis (BB-12) is added to YoPlus yogurt (also in
USANA Probiotic -- see Results table) and Bifidobacterium
animalis DN-173 010 (trade named "Bifidus regularis")
is in Dannon's Activia yogurt. Clinical trials on both
probiotic strains have shown that they shorten the transit time of food in the
bowel; speeding up the time for food to pass through the bowel may benefit
people with constipation, but it is not necessarily beneficial for people who
complain of frequent or loose stools. A dosing study with BB-12 showed looser
stools with increasing probiotic dose (which ranged from 100 million to 100
billion cells per day) (Larsen 2006).
A study of yogurt containing Bifidobacterium
animalis DN-173 010 (as in Activia) including yogurt
starter cultures S. thermophilus and L. bulgaricus found
that after 4 weeks of eating two cups (each 125 grams, non-flavored) daily, 57%
of people with IBS involving constipation reported adequate symptom relief.
However, among those in a control group eating a similar yogurt product which
had been heated to kill the probiotic organisms, an almost equal amount of
people (54%) reported relief, indicating no significant benefit of the
probiotic over the non-probiotic. After eating the products for 8 weeks, 68% of
those in the non-probiotic group reported adequate relief compared to just 46%
of those who had taken the probiotic; and at 12 weeks this increased to 76% for
the non-probiotic eaters and remained at 46% for the probiotic eaters. The
researchers concluded that people with IBS may benefit from regular consumption
of a fermented dairy product, like yogurt, but the addition of this particular
probiotic would not be expected to provide further benefit (Roberts, BMC Gastroent 2013).
Some clinical studies suggest that prebiotics may improve symptoms
of IBS, but results have been mixed. For more information about
fructo-oligosaccharides and other prebiotics see the Prebiotics section of the review.
In children:
A study of children (aged 5 to 14) with IBS showed that
taking Lactobacillus GG (a strain of Lactobacillus
casei which is used in Culturelle — see Results table) (3 billion cells twice per
day for 8 weeks) significantly reduced the frequency and severity of abdominal
pain (Ruggiero 2010). The number of episodes of pain
per week decreased from 3.4 to 1.6 during treatment (a decrease of 4.0 to 3.2
was seen in the placebo-treated group). Episodes of pain fell even further
during the follow-up period (8 weeks after therapy) to 0.9 per week in the
probiotic group (1.6 in the placebo group). The treatment was well tolerated
and no adverse effects were reported.
Constipation and infrequent bowel movements without IBS
A study in
healthy adults without IBS but who reported a low frequency of bowel
movements (2 - 4 days per week) and abdominal discomfort, such as pain
or bloating, found that a dose of 1 billion cells of Bifidobacterium
animalis subsp. Lactis (BB-12®, Chr. Hansen
A/S ) taken for one month (one capsule daily with breakfast) significantly
increased the frequency of bowel movements, but did not improve abdominal pain
or bloating, compared to placebo. A third group, who took a higher dose (10
billion cells of BB-12®) daily had similar results. The study was
funded by the maker of the probiotic (Eskesen, Br J Nutr 2015). BB-12® is found in the USANA
Probiotic (see Results table).
As noted above, probiotics tend to speed up movement of food through the
bowel and may, therefore, help people with constipation. A study in
adults in Italy with chronic constipation found that taking a tablet providing
100 million cells of Lactobacillus reuteri DSM 17938 30
minutes after eating, twice per day for 4 weeks, significantly increased the
number of bowel movements per week from a mean of 2.68 to 5.28. This was a
statistically significant increase compared to the placebo group, which
experienced a smaller increase, going from 2.89 to 3.89 bowel movements per
week. Both groups reported an increase in softer stools, but there was no
statistically significant difference between the groups. None of the patients
reported adverse experiences (causing discomfort and/or interrupting usual
activity) (Ojetti, J Gastrointest Liv Dis,
2014).
Bloating, gas and abdominal pain with or without IBS
A clinical study compared the effects of a daily probiotic to that of placebo
in several hundred adults who had regularly experienced abdominal discomfort
and bloating for at least three months but who did not have IBS or other
gastrointestinal disease. After four weeks of treatment, both groups showed
significant but roughly equivalent decreases in the severity of abdominal
discomfort and of bloating, The probiotic group had more days free of bloating,
but not days free of abdominal discomfort (Ringel-Kulka, Am J Gastroent 2016).
The probiotic given was 1 billion cells daily of B. infantis 35624
(Align). The study was designed and sponsored by Proctor & Gamble,
with the hope that Align would reduce the severity of
abdominal discomfort and bloating in people without IBS as it had been shown to
do in one study in patients with IBS (see Whorwell 2006 above), but this was
not the case.
A study in young, healthy children in Mexico found that 1
billion cells of Bacillus coagulans GBI-30, 6086 (GanedenBC30 by Ganeden Inc. -- found in Schiff
Digestive Advantage Gas Defense Formula and other products tested in
this review) taken daily for three months modestly reduced the incidence of
flatulence (gas) compared to placebo. In the same study (which was supported by
Ganeden), this strain was also found to reduce certain symptoms of upper
respiratory infections — see details in Colds and acute respiratory infections below.
Some (but not all) studies suggest that proton-pump
inhibitor drugs (PPIs), such as Prilosec (omeprazole), which reduce
stomach acid, can cause people to experience bloating and other unpleasant
bowel symptoms. A potential explanation is that the lowered acidity in the gut
results in an overgrowth of bacteria in the small intestine. A
placebo-controlled study in people who began taking the PPI pantoprazole
(Protonix) (40 mg per day) to treat gastroesophageal reflux showed that taking
a probiotic twice daily appeared to prevent the onset of bowel symptoms. After
about 3 months on the PPI, approximately 45 to 50% of patients taking the
placebo (rather than the actual probiotic) reported bloating and flatulence,
while these symptoms were reported by only about 10% of those taking the
probiotic. The differences were statistically significant. There was, however,
no statistically significant difference between the groups in terms of reported
abdominal pain. (Compare, Digestive and Liver Dis
2015). The probiotic consisted of 12 billion cells of Lactobacillus
paracasei F19 (Gene-filus F19, Siffra, Italy) from a sachet dissolved
in water, twice a day, 3 days a week. The study was funded by the company,
Siffra.
A study in Belgium among 55 men and women (average age 40)
with IBS and/or functional dyspepsia (chronic
gastrointestinal symptoms such as upper abdominal pain, gas, bloating and
indigestion), about half of whom were taking a proton-pump inhibitor, found
that a higher percentage of those who took a probiotic containing two
spore-forming organisms (twice daily with meals for two months) had clinical
improvement (> 0.7 point decrease on a 4 point scale) in gastrointestinal
symptoms after eating than those who took a placebo. Forty-eight percent (12 of
25) of those who took the probiotic had clinical improvement compared to 20% (6
of 30) among the placebo group, and improvements were similar whether or not
participants were using proton-pump inhibitors. Side effects were generally
mild, and included diarrhea and stomach pain, although one there was one
incident of skin infection in a participant taking the probiotic, resulting in
discontinuation of use. Each serving of the probiotic contained 10 billion
cells of a combination of Bacillus coagulans MY01 and Bacillus
subtilis MY02 -- provided by My Health, which funded the study (Wauters, Lancet Gastroenterol Hepatol 2021).
Acute diarrhea and abdominal pain
A study in India among 60 men and women experiencing acute
diarrhea and abdominal pain found that those who took a probiotic (two billion
cells of B. coagulans LBSC) three times daily starting within
48 hours of onset of diarrhea (cause not specified) averaged a 70% reduction in
severe abdominal pain within one day compared to a 3.3% reduction among those
who took a placebo. After two days of supplementation, loose stools also
decreased more in the probiotic group than the placebo group. After five days,
all of those taking the probiotic had complete remission of diarrhea and
abdominal pain, compared to only 3% of those who took the placebo. It should be
noted that the authors of the study work for the company that supplied the
probiotic (Maity, Eur J Clin Pharmacol 2018).
As discussed earlier, B. coagulans strains have also been found
to reduce symptoms in diarrhea-predominant IBS. Several products in this Review
include strains of B. coagulans, as noted in the second column of
the Results table.
Ulcerative colitis
Clinical guidelines for the treatment of ulcerative colitis in
countries such as South Korea and Canada currently recommend against the
use of probiotics for treatment and/or to induce or maintain "complete
remission" (Choi, Intest Res 2017; Bressler, Gastroenterology 2015).
These positions were taken despite an analysis showing that three randomized,
controlled trials among people with active ulcerative colitis receiving
standard medications found that those who took the probiotic VSL#3 (3.6
trillion cells daily) for an average of 3 months had a higher remission rate
compared to those who took placebo (43.8% vs. 24.8%, respectively) (Mardini Inflamm Bowel Dis 2014). The
American Gastroenterological Association makes no recommendation for the use of
probiotics for ulcerative colitis (Grace, Gastroenterology 2020),
cautioning that their use risks delaying proven effective therapy with the
potential for worsening symptoms or complications (Ko, Gastroenterology 2019).
a study among 31 patients with ulcerative colitis who were given the same daily
dose of VSL#3 as part of their care at the Cleveland Clinic found that after 8
months of treatment, 25 had discontinued taking VSL#3, mostly due to recurrence
of symptoms; two discontinued due to adverse effects such as bloody bowel
movements, severe gas, bloating and constipation. The six patients who
continued taking VSL#3, for an average of six more months, did not have
significant improvements in symptoms (Shen, Aliment Pharmacol Ther 2005).
This study was not blinded or placebo-controlled; however, the authors noted
that it was intended to incorporate the "promising results" from
previous double-blind, placebo-controlled studies "into routine clinical
practice."
A study among 40 men and women with chronic relapsing pouchitis (a
common complication of ileo-anal pouch surgery needed as a result of ulcerative
colitis) found that those who took VSL#3 (300 billion cells, twice daily) for
nine months had significantly fewer relapses than those who took a placebo.
Among those who took the probiotic, three people (15%) experienced a relapse,
while 20 people (100%) of those in the placebo group experienced relapse (Gionchetti, Gastroenterology 2000). In
adults and children with pouchitis, the American Gastroenterological
Association recommends the use of the eight-strain combination found in VSL#3 (Grace, Gastroenterology 2020).
[Note: Be aware that the VSL#3 formula used in these studies
is now sold as Visbiome by ExeGi Pharma (it is also sometimes
referred to as the "original De Simone formulation", after its
inventor). The formula currently sold as VSL#3 (by Pharmacueticals/Alfasigma
and distributed in the U.S. by Sigma-Tau Healthscience USA) is not the same as
that used in these studies. See What to Consider When Buying for more
details.]
Abdominal surgery
Giving a probiotic around the time of major abdominal surgery (such as
colorectal or liver resection) may increase the rate of
re-admission to the hospital after surgery, according to a recent study.
Patients in the study were given one capsule of the formula currently sold
as VSL#3 by Alfasigma or a placebo capsule, just before
surgery and then twice daily (up to 15 total doses) before discharge. The
probiotic did not increase survival rates or reduce post-operative infections
compared to placebo. Those who took VSL#3 were more
likely to need to be re-admitted to the hospital within 30 days of
surgery than those who took the placebo (16.4% vs 4.4%) due to dehydration as a
consequence of diet intolerance and/or diarrhea, and readmission rates were
still significantly higher in the probiotic group 60 days after surgery (19.4%
vs 5.9%). Each capsule of VSL#3 contains 112.5 billion cells
per capsule of the following strains: Bifidobacterium breve,
Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus,
Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus and Streptococcus
thermophilus (Franko, J Am Coll Surg 2019).
[As noted above, the formula now sold as VSL#3 by Alfasigma is
not considered to be the original formula used in older studies.]
Lactose intolerance
Lactose intolerance is a form of lactose maldigestion in which individuals who
produce lower amounts of lactase enzyme develop gas, abdominal bloating and
pain, and even diarrhea and nausea, after consuming milk or dairy products.
Fermented milk products, such as yogurt and kefir, produce fewer symptoms of
lactose intolerance than milk containing the same amount of lactose, likely due
to the organisms in their cultures which contain lactase enzyme. However, it
has not been well demonstrated that taking a probiotic supplement, or adding
probiotics to milk products, improves lactose digestion (Levri, J Fam Pract 2005).
A small, 4-week study sponsored by the makers of the probiotic DDS-1 (a strain
of Lactobacillus acidophilus) found that taking a capsule with 10
billion cells at breakfast daily reduced abdominal symptoms such as diarrhea,
cramping and vomiting compared to placebo in people with reported symptoms of
lactose intolerance (although they were not clinically diagnosed). However, it
failed to reduce gas (flatulence) and bowel sounds and did not improve lactose
digestion (based hydrogen breath tests). (Pakdaman, Nutr J 2016)
(Also see ConsumerLab.com's review of Products for Lactose Intolerance —
Lactase Enzymes and Lactose-free Milks)
Functional abdominal pain in children
A study of children (ages 6 to 16) with functional abdominal pain given Lactobacillus
reuteri DSM 17938 (200 million cells per day) for 4 weeks showed
reduced intensity of abdominal pain in the treated children compared to those
given placebo (Romano, J Paediatr Child Health
2014).
Colic in infants
Lactobacillus reuteri DSM 17938 has also shown benefit in preventing
colic in newborns and reducing colic in some infants. A 3-month study of
healthy newborns given 100 million cells per day (from 5 drops of an oil
formulation sold as Gerber Soothe Colic Drops) found that, compared to
placebo, infants receiving the probiotic drops had a significant decrease in
crying time (38 minutes vs. 71 minutes per day), regurgitation (2.9 vs. 4.6
times per day) and an increase in frequency of stools (4.2 vs. 3.6 times per
day). The declines in crying time and regurgitation were significant within the
first month of treatment. Treated infants also had significantly fewer visits
to emergency rooms for colic, fewer lost working days among parents, and lower
use of other treatments for abdominal discomfort (Indrio, JAMA Pediatr 2014).
While this study showed benefit in preventing colic in
newborns, a study in infants with colic severe enough for parents to seek
emergency care found that the same probiotic, given for one month, provided no
more benefit than placebo. Although crying and fussing time decreased in both
the probiotic- and placebo-treated groups, among formula-fed infants
"fussing" decreased more among those given placebo than among those
given the probiotic (Sung, BMJ 2014). In
infants with colic seen routinely as outpatients (non-emergency), a study
giving the same probiotic and dosage as the earlier studies for 30 days found
crying decreased from about 300 minutes per day to just 75 minutes per day,
which was significantly better than a decrease to 148 minutes per day among
infants who were given placebo drops. The probiotic-treated infants also showed
a significantly greater decrease in a marker of intestinal inflammation, known
as fecal calprotectin, than the placebo group. (Savino, J Pediatr 2017).
Diverticular disease
A study in 210 people with symptomatic uncomplicated diverticular disease
(SUDD) found that treatment with a probiotic (Lactobacillus casei subsp.
DG, 24 billion organisms) was nearly as effective as treatment with the drug
mesalazine (1.6 grams) in reducing recurrence of symptoms, and combined
treatment was more effective than either alone. The treatments were given for
10 consecutive days each month for one year. Over the year, the percentage of
patients with recurrence of symptoms (abdominal pain lasting at least 24 hours)
was 46% among patients who received only placebo, 14.5% among those who
received the probiotic, 13.7% among those who received mesalazine only, and 0%
for those receiving combination treatment (Tursi, Aliment Pharm Ther 2013). Mesalazine is
believed to work by controlling inflammation while the probiotic may restore
organisms in the colon.
A study in Italy among 88 middle-aged people seen in a
hospital with acute uncomplicated diverticulitis found that 10 days of
probiotic treatment (Lactobacillus reuteri ATCC PTA 4659 from
Biogaia AB, 500 million organisms per capsule taken 30 minutes after food,
twice daily) started along with 5 days of antibiotic treatment (ciprofloxin and
metronidazole) resulted in better outcomes than the antibiotic treatment plus a
placebo capsule. At 3, 5, 7, and 10 days after initiation of therapy, the
probiotic group consistently showed greater decreases in pain -- about 2 points
lower on a pain scale of 1 to 10 at each time point. In addition, the probiotic
group experienced a greater decrease in CR-P, a blood marker of inflammation,
and stayed, on average, one day less in the hospital (Petruzziello, Int J Colorect Dis 2019). (Note:
This probiotic does not seem to be in any currently marketed supplements.)
Diarrhea caused by antibiotics, viral infection, or
chemotherapy
Antibiotic-associated diarrhea:
Diarrhea is a common side effect of antibiotics, occurring in 5% to 25% of
patients, particularly older individuals (Högenauer, Clin Infect Dis 1998). Many
studies, although not all, have shown probiotics reduce the risk of diarrhea
associated with antibiotic treatment. As discussed below, better results have
been achieved from products containing multiple strains of probiotics including
yeast (Saccharomyces) as well as Lactobacillus and Bifidobacterium. Higher
doses (close to ten billion or more cells daily) may be preferable. It appears
best to begin probiotic treatment around the time of starting antibiotics
(although take probiotics at least 2 hours after the antibiotic) and continue
the probiotic for at least one week after antibiotic therapy ends. However, one study found that continuing a probiotic for one
month after ending antibiotics delayed the restoration of normal bacteria in
the gut by a month (Suez, Cell 2018) — so it may be better to end
probiotics sooner, rather than later, after stopping antibiotics.
Evidence is too inconclusive to recommend probiotics for treating or
preventing Clostridioides
difficile (C. difficile) infection, which accounts for about one
third of cases of antibiotic-associated diarrhea.
A 2012 review of over 60 published studies concluded that probiotic use was
associated with an overall 42% lower risk of developing diarrhea due to
antibiotic treatment (Hempel, JAMA 2012). There was a 36% lower risk
of diarrhea across studies specifically using forms of Lactobacillus and
a 52% lower risk of diarrhea across studies using the probiotic yeast Saccharomyces.
Looking at specific strains, the probiotic yeast Saccharomyces
boulardii (a strain found in FloraStor and FloraStor
Kids) has been shown to be helpful in preventing antibiotic-induced
diarrhea (Cremonini 2002) as well as other
gastrointestinal symptoms and changes in the microbiota at a dose of 500 mg
twice daily during a week of antibiotic treatment and for a week afterward (Kabbani, Gut Microbes 2017).
A probiotic drink (Actimel — also sold as DanActive)
containing Lactobacillus casei, Lactobacillus bulgaricus,
and Streptococcus thermophilus consumed twice daily during
antibiotic treatment and for a week thereafter, significantly decreased the
risk of developing diarrhea (Hickson, 2007). A study in China among
hospitalized adults (aged 50 to 70) showed that capsules containing large doses
of proprietary strains of Lactobacillus acidophilus and Lactobacillus
casei significantly reduced antibiotic-associated diarrhea (Gao, 2010).
Another study in China showed greater overall efficacy
with a higher dose of a probiotic than a lower dose. In this study of
hospitalized adults aged 30 to 70, the incidence of antibiotic-associated
diarrhea was reduced from 24.6% among those who received placebo to about half
that amount (12.5%) among those receiving 17 billion probiotic bacteria per
day. A group receiving only 4 billion bacteria per day had a non-significant
decrease to 19.6%. The higher dose probiotic treatment also significantly
reduced the incidence of symptoms, particularly abdominal pain (19.2% placebo
vs. 2.5% high-dose) (Ouwehand, Vaccine 2013).
A capsule containing the probiotic was taken 2 hours after breakfast (the
antibiotic was taken with breakfast) on each day of antibiotic treatment and
for 7 additional days. The probiotic used in the study consists of a
combination of four bacteria in equal parts marketed as HOWARU Restore.
See What to Consider When Using — Dosage for
more about this probiotic.
A study among healthy adults in Canada given the antibiotic
amoxicillin-clavulanic acid for one week evaluated the effect of giving a
probiotic vs. placebo during the time of antibiotic therapy and for one week
after the antibiotic. Both groups experienced significant increases in
looseness and frequency of bowel movements — however, among those who
experienced diarrhea (25% and 33% of the probiotic and placebo groups,
respectively), the duration of diarrhea was one day shorter in the probiotic
group compared to the placebo group (2.7 days vs. 3. 7 days, respectively),
which is similar to findings from other studies. The beneficial effect of the
probiotic was most evident during the week after antibiotic therapy. The
probiotic was Lacidofil® Strong (made by Lallimand, with each dose consisting
of 3.8 billion cells of Lactobacillus rhamnosus R0011 and 200
million cells of Lactobacillus helveticus R0052, This was
taken twice a day with breakfast and dinner, while the antibiotic was taken 30
minutes before those meals. (Evans, Br J Nutr 2016). A similar study using
the same probiotic but at half the dose did not find a benefit (Song, J Korean Med Sci 2010).
A major, well-controlled study of hospitalized patients age
65 and older in the U.K. who were receiving oral or parenteral antibiotics
did not find probiotics helpful in preventing
antibiotic-associated diarrhea or Clostridium difficile diarrhea.
However, the study had a major weakness: probiotic therapy was not
started at the initiation of antibiotic treatment, but 7 days later. The study
used a probiotic containing a blend of Lactobacillus acidophilus CUL60
(NCIMB 30157) and two strains of Bifidobacterium bifidum CUL20
(NCIMB 30153) (a total of 60 billion bacteria per day) given daily for 21 days
(Allen, Lancet 2013).
Many studies have focused on probiotics specifically for
preventing Clostridioides difficile (C. difficile) infection, which
is typically caused by exposure to broad-spectrum antibiotics and can be
life-threatening. Although some research has shown benefit of certain
probiotics for this indication, most guidelines do not recommend probiotics for
this indication due to inconsistent trial results, lack of clear evidence of
benefit of a particular strain/combination of strains, and the cost of
treatment.
A review of 20 studies in 2012 found that giving
probiotics reduced the risk of C. difficile-associated diarrhea by
66% compared to not giving probiotics. Products with multiple species of
probiotic organisms tended to have larger effects (75% risk reduction) than
those using single species (50% risk reduction), although the difference was
not statistically significant. People treated with probiotics reported fewer
adverse events (e.g., cramping) than those who received placebo or no
treatment, highlighting the safety of probiotics for this use (Johnston, Ann Int Med 2012).
The results were similar in trials of adults and children. Each of the trials
used at least 10 billion organisms daily, but there was no significant
difference between higher (>10 billion organisms) and lower doses (10
billion organisms or less). It was unclear which, if any, strain or combination
of strains was most beneficial.
Another review published in 2017 of 19 studies similarly found giving
probiotics reduced the risk of C. difficile-associated diarrhea by
more than 50% in hospitalized adults. Most importantly, the study showed the
importance of starting probiotic therapy early: Starting probiotics within the
first 2 days of antibiotic initiation resulted in a 68% reduction in risk
of C. difficile infection as compared to 30% reduction if
started after the first 2 days of antibiotic initiation (Shen, Gastroenterology 2017).
Despite this evidence, the American College of
Gastroenterology recommends against the use of probiotics for
treating or preventing C. difficile infection since the
evidence of benefit comes mainly from small studies that assessed C.
difficile infection as only a secondary outcome. It states that, given
the lack of high-quality studies showing benefit, there is insufficient
evidence to justify use of probiotics for C. difficile, especially
since formulations recommended for this condition typically cost about $30 to
$100 per month, are taken for extended periods of time, and are usually not
covered by insurance (Kelly, Am J Gastroenterol 2021).
The American Gastroenterological Association provides more nuanced
recommendations, stating that, while some patients may benefit from taking
certain combination of probiotics, probiotics may not be appropriate for those
with low risk of developing C. difficile, those who are immunocompromised,
or those who wish to avoid the cost of probiotic treatment (Su, Gastroenterology 2020).
Diarrhea from infections:
Lactobacillus GG (see Culturelle in the Results table) and Lactobacillus
reuteri have been shown to reduce the duration of diarrhea due to
certain infections in infants and young children, but not adults. In children, Lactobacillus GG (5.2 billion
cells/day) has also been effective in reducing antibiotic-associated diarrhea
in combination with Bifidobacterium lactis (Bb-12) [see USANA
Probiotic in the Results table] (5.9 billion cells),
and Lactobacillus acidophilus (La-5) (8.3 billion cells),
given in yogurt. An Australian study investigated the effects of this probiotic
yogurt (sold in Australia as Vaalia from Parmalat) in children
being treated with antibiotics for ear and throat infections. They were given
the yogurt twice a day starting with commencement of antibiotic therapy and for
one week after therapy. Only one of the 36 children experienced mild diarrhea,
in contrast to 21 of 34 children given regular yogurt. None of the children in
the probiotic group experienced severe diarrhea, in contrast to 6 out of 34
children given regular yogurt (Fox, BMJ Open 2015). [Note: Although Vaalia is
not sold in the U.S., other products containing all or some of the species
(although at different doses) are Culturelle, and USANA
ProBiotic.]
Diarrhea associated with chemotherapy: Lactobacillus
rhamnosus GG taken twice daily (10 to 20 billion cells total per day)
as a capsule or capsule contents dissolved in cold milk or juice has also been
shown to reduce the risk of chemotherapy-induced diarrhea (Osterlund, B J Cancer 2007).
A dosing study (Saxelin 1991) using the bacteria in Culturelle (Lactobacillus GG)
found that a daily dose of 1.5 billion cells was not able to colonize the gut,
but a much larger dose, 15 billion cells, was. (Culturelle tested
by ConsumerLab.com in this Review provides 10 billion cells in its suggested
daily serving of one capsule.)
Feeding intolerance and enterocolitis in infants:
Lactobacillus reuteri DSM 17938 (100 million cells daily) given
shortly after birth to preterm infants resulted in decreases in feeding
intolerance and the duration of hospitalization compared to placebo, as well as
a 40% (but not statistically significant) decrease in necrotizing enterocolitis
(Rojas, Pediatrics 2012).
The American Gastroenterological Association suggests the use of probiotics
such as Lactobacillus reuteri DSM 17938 probiotics in the
prevention of necrotizing enterocolitis (NEC) in preterm infants less than 37
weeks gestational age and low birth weight (Grace, Gastroenterology 2020).
Traveler's diarrhea
Studies using oral probiotics have yielded both positive and negative
results. Lactobacillus GG reduced the risk of traveler's
diarrhea by 47% in a study involving 245 people who traveled to 14 world-wide
geographic regions (Hilton, 1997). Saccharomyces
boulardii reduced the likelihood of traveler's diarrhea by 13% (using
250 mg per day) to 26% (using 1 gram per day) in a study of 3,000 Austrian
tourists who traveled in northern Africa, the Middle East and Far East. This
study had a high drop-out rate of 34% (Kollaritsch, 1993). Three weeks of Lactobacillus
fermentum KLD or Lactobacillus acidophilus failed to
prevent traveler's diarrhea in a study of 282 British soldiers deployed to
Belize (Katelaris, 1995). A
combination of Lactobacillus acidophilus and Lactobacillus
bulgaricus failed to prevent traveler's diarrhea in 50 travelers to
Mexico (de dios Pozo-Olano, 1978). The effectiveness
of individual probiotic species for traveler's diarrhea may vary depending on
the probiotic species used and the travel destination.
Helicobacter pylori (H. pylori)
infection
Although some evidence suggests that taking probiotics containing Lactobacillus, Bifidobacterium or Saccharomyces species
in addition to antibiotics may help to increase eradication of H.
pylori (a causative agent of stomach ulcers) (Fashner, Am Fam Physician 2015), or enhance
tolerance to antibiotics for H. pylori (which might improve
adherence to treatment), most of the studies examining probiotics for H.
pylori infection have been conducted in China and are not well
designed. Furthermore, questions remain as to which probiotic strain may be
most beneficial, what dose and duration of treatment would be optimal for
improving eradication, and which people would be most likely to benefit from
probiotic add-on therapy. For this reason, the American College of
Gastroenterology does not recommend for or against the use of probiotics with
antibiotics to treat H. pylori infection (Shah, Gastroenterology 2021).
COVID-19 (SARS-CoV-2)
There is currently no direct evidence that a probiotic can prevent or
treat COVID-19, the disease
caused by the coronavirus SARS-CoV-2). It is possible, based on indirect
evidence, that probiotics could play some role in treatment, but this remains
to be demonstrated.
Researchers in China have reported imbalances in gut bacteria in patients with
COVID-19, particularly decreases in Lactobacillus and Bifidobacterium (another
lactic-acid producing bacteria). The researchers suggested the use of
prebiotics or probiotics "to regulate the balance of intestinal microbiota
and reduce the risk of secondary infection due to bacterial
translocation," although they did not specify exact strains nor did they
appear to test this hypothesis (Xu, Zhejiang Da Xue Xue Bao Yi Xue Ban 2020).
Some probiotic bacteria, especially lactic acid-producing bacteria such
as Lactobacillus acidophilus, Lactobacillus plantarum and Enterococcus
faecium, have immunomodulatory and antiviral activities that have been
shown in preliminary research to help protect against other viruses that cause
respiratory or intestinal symptoms (Rejish, Trop Anim Health Prod 2010). For
example, a study in mice found that giving Lactobacillus plantarum L-137
before infection with an H1N1 influenza virus decreased viral load in the lungs
and increased survival, possibly by increasing blood levels of beta interferon (Maeda, Int Immunopharmacol 2009).
Laboratory studies suggest that certain probiotic strains may also help to
protect against coronaviruses known to cause gastrointestinal symptoms. For
example, two Lactobacillus strains, L. casei Shirota
and L. plantarum PCA236, have been shown to have antiviral
activity against a coronavirus known as transmissible gastroenteritis virus
(TGEV) (Maragkoudakis, Int J Food Microbiol 2010).
Another study showed TGEV attached to the surface of the probiotic strain Enterococcus
faecium NCIMB 10415, which, the researchers noted, could be a
mechanism by which the probiotic might trap virus and to prevent infection (Weidong, Arch Virol 2013).
As discussed below, supplementation with various strains of Lactobacillus, Bacillus and Bifidobacterium have
been shown to reduce the incidence of colds and symptoms of viral upper
respiratory infections such as coughing and fever. Upper respiratory symptoms
are a component of COVID-19, although a more serious component is lower
respiratory (i.e., lung) involvement. A
placebo-controlled trial to evaluate the effects of a commercially available
probiotic (Culturelle -- Lactobacillus rhamnosus GG) on the
transmission of COVID-19 among household members in which one member has
contracted the virus is scheduled begin in the summer of 2020. The study will
be conducted by researchers at Duke University and results of the trial are
expected in May 2022 (Clinicaltrials.gov 2020).
Colds and acute respiratory infections
Although some research suggests that probiotics may help prevent colds and
acute respiratory infections, other studies have found no benefit, particularly
in adults, and it is unclear which, if any, specific probiotic strain and dose
works best.
Adults:
Among healthy adults in Sweden given 1 billion viable cells daily of a
combination of Lactobacillus plantarum HEAL 9 (DSM 15312)
and Lactobacillus paracasei 8700:2 (DSM 13434) for 12 weeks,
55% experienced colds compared to 67% of those who received placebo (Berggren, 2011). These
probiotic strains can be found in Metagenics UltraFlora® Immune Booster.
The number of days with cold symptoms was also lower (6.2 days vs. 8.6 days for
the placebo). However, a subsequent study in Germany
using this same probiotic and dosage did not reduce the number
of days with cold symptoms, the severity of symptoms, or the incidence of
recurring colds compared to placebo. Although there was a statistically
significant reduction in the number of colds compared to placebo, the
difference was likely too small to have a practical benefit (1.24 vs 1.36
colds). The study was conducted during three consecutive cold seasons among 869
adults. The most common side effects associated with the probiotic were gas,
stomach pain, nausea, and headache (Ahren, J Nutr 2020).
A study among 232
healthy adults showed that supplementing with Bifidobacterium animalis subsp lactis Bl-04
(at least 2 billion cells per day) for 4 weeks prior to exposure to a
rhinovirus (a virus that causes colds) did not reduce the
number of patients who developed a cold within two weeks of exposure compared
to the placebo group. Probiotic supplementation also did not reduce
the duration of illness or the severity of symptoms compared to placebo among
those who became sick (Turner, EClinicalMedicine 2021).
Children:
Giving children (aged 3 to 5) in China a combination of Lactobacillus
acidophilus NCFM (5 billion cells per day) and Bifidobacterium
animalis subsp lactis Bi-007 (5 billion cells per day) versus placebo
for 6 months (November to May) was shown to reduce the incidence of fever by
73%, coughing by 62%, runny nose by 59%, and also reduced the duration of these
symptoms by 48% (Leyer, 2009). Lactobacillus
acidophilus alone (10 billion cells per day) was also effective but
not as effective as the combination product. A study among 80 children in
Mexico (aged 6 to 8) found that 1 billion cells of Bacillus coagulans GBI-30,
6086 (GanedenBC30 by Ganeden Inc., which funded the study) taken daily for
three months modestly reduced the incidence (but not the severity) of certain
symptoms of upper respiratory tract infections such as nasal congestion,
including bloody nasal mucus, itchy nose, and hoarseness, and decreased the
duration of headache, red eyes, and fatigue, compared to placebo. The
researchers also measured the probiotic's effects on gastrointestinal symptoms
in the children and found that, compared to placebo, it reduced the incidence of flatulence (gas)
-- but not other symptoms such as constipation or diarrhea (Anaya-Loyola, Physiol Behav 2019). (GanedenBC30 is found in Schiff Digestive
Advantage Gas Defense Formula.)
Short-term use of probiotics may also help. In a study in Ukraine, children
ages 3 to 12 were started on a daily dose of Lactobacillus acidophilus DDS-1
(1 billion cells) and Bifidobacterium lactis UABLA-12 (4
billion cells) given as a powder (mixed with water or juice) which included a
fructooligosaccharide prebiotic (50 mg) on the first day that a household
member appeared to be sick. (The powder is sold in capsules as UP4 Probiotics
with DDS-1, UAS Lifesciences — see Results table). Treatment continued for
two weeks or until the end of any infection the child developed. In all, 57% of
children in the probiotic group and 65% of those in the placebo group developed
acute respiratory infections. Although this was not a statistically significant
difference, the duration of infection was significantly shorter (median
duration 5 days vs. 7 days) and the severity of infection was significantly
less in the treated vs. placebo group (Gerasimov, Eur J Clin Nutr 2015).
Throat infection
There is evidence that certain probiotic strains may protect against some types
of bacterial and viral throat infections. One small study found that children
taking a daily probiotic lozenge containing 1 billion colony-forming units
of S. salivarius K12 (Bactoblis®, now known as BLIS K12®) for 3 months
had significantly fewer episodes of throat infection than children who were not
given the lozenge. Incidence of viral oropharyngeal infections in treated
children was reduced by 80%, and streptococcal infections by 96% (Pierro Drug Healthc Patient Saf 2014). A gum
containing BLIS K12® (CulturedCare® Probiotic Gum by Prairie
Naturals) has been approved and licensed to be sold in Canada with the
allowable claim that it promotes oral health through recolonization of the oral
cavity after antibiotic treatment and, following microbial rinsing,
it helps reduce halitosis (bad breath) by reducing volatile sulfur
compound levels. In 2014, a lozenge containing BLIS K12® received approval in Canada to
use these same health claims, as well as the claim that it can help reduce the
incidence of Streptococcal pharyngitis and/or tonsillitis (Health Canada 2018). CulturedCare®
Probiotic Gum and supplements containing BLIS K12® are
also available for sale in the U.S. In 2015, the U.S. FDA informed the
maker of BLIS K12 that it does not object to the company's
self-affirmation that the product be Generally Recognized as Safe.
Dental Plaque
Another strain of S. salivarius (BLIS M18, Blis
Technologies) has been shown, when taken as a lozenge, to reduce dental plaque
in children (Burton, J Med Microbiol 2013). It was
further shown to specifically reduce the development of a type of plaque known
as black stains (which is high in insoluble iron, creating a dark color) in a
study of 54 children who had undergone a dental cleaning to remove black
stains. Among those who then took 1 billion cells of BLIS M18 (as a slowly
dissolving tablet) daily for three months, only 21.2% developed black stains
versus 50% of those who did not receive the probiotic. Three months after
supplementation ended, only 32.1% of those who took the probiotic developed
black stains compared to 53.8% of those who had not (Bardellini, Oral Health Prev Dent 2020).
Periodontitis (gum disease)
A lozenge containing probiotics may be helpful in
treating chronic periodontitis - inflammation around the teeth caused by
microbial infection, which can result in pockets between the teeth and gums. In
a 12-week study in Turkey, 30 otherwise healthy individuals with adult chronic
periodontitis dissolved a probiotic lozenge in their mouth twice a day
following an initial dental scaling and disinfection with chlorhexidine
solution. All patients, including those given placebo, experienced
improvements, but those using the probiotic had significantly greater reduction
in the depth of pockets around affected teeth and gain in tooth attachment to
ligaments in moderate and deep pockets. The probiotic-treated group also had a
greater reduction in several parts of the mouth of Porphyromonas
gingivalis, a bacterium considered a keystone in the onset of chronic
periodontitis. The probiotic treatment was most effective in patients with
moderate to severe periodontitis. The lozenge contained 100 million cells of
each of two strains of Lactobacillus reuteri DSM17938 and ATCC
PTA5289 (Prodentis from
BioGaia, Sweden, sold in the U.S. and Canada as GUM PerioBalance (Teughels, J Clin Periodont 2013).
Similarly, a study among 41 men and women in Brazil who,
twice a day, used a lozenge containing 1 billion cells of B. lactis HN019
(HOWARU Bifido LYO 40 DCU -S, DuPont Danisco, Finland) for one month after a
deep cleaning treatment for chronic periodontitis (scaling and root planning)
had significantly greater reduction in the depth of pockets around affected
teeth and gain in tooth attachment to ligaments, as well as fewer bacteria and
certain markers of inflammation associated with periodontitis in the fluid
around the gums compared to those who took a placebo after the deep cleaning.
(This strain is also found in capsule form in HOWARU Restore for
other uses.) (Invernici, J Clin Periodontol 2018).
Vaginal bacterial infections
A daily dose of at least 1 billion cells each of L. rhamnosus GR-1
and L. fermentum RC-14 (now called Lactobacillus
reuteri, RC-14) taken orally has been shown to reduce colonization of the
vagina by potential pathogenic bacteria and yeast in women with asymptomatic
bacterial vaginosis (Reid FEMS Immunol Med Microbiol 2003).
[These two strains can be found in two products that were tested and Approved
by ConsumerLab in 2015, Jarrow Formulas femdophilus and RepHresh
Pro-B Probiotic.] However, this same probiotic combination was not
shown to have any effect on the ratio of pathogenic microbes to harmless lactobacillus bacteria
in the urinary tracts and rectovaginal skin areas of healthy pre-menopausal
women in a more recent, placebo-controlled study. The women consumed 1 billion
cells of the combination twice daily for 40 days. The probiotic species were
not found in any collected samples, leading the researchers to
conclude that "it is unlikely that the probiotic organisms had a direct
effect on the lower urinary tract." It was also noted that the method of
detection of bacterial species was more rigorous in this study as compared to
the earlier study by Reid. On the other hand, a weakness of this study is that
the quality of the probiotic product was not confirmed (Wolff, Int Urol Nephrol 2019).
Vaginal suppositories (which are inserted and not considered supplements in the
U.S.) containing Lactobacillus organisms (including L.
rhamnosus, L. gasseri, L. fermentum LF10 and L. acidophilus LA02)
have shown therapeutic benefit (Vicariotto, J Clin Gastroenerol 2012; Pendharkar, BMC Infect Dis 2015).
Vaginal yeast infections are a common occurrence after antibiotic treatment,
but neither an oral probiotic (containing Lactobacillus rhamnosus and Bifidobacterium
longum), nor a vaginally inserted probiotic (containing L.
rhamnosus, L. delbrueckii, L. acidophilus, and Streptococcus
thermophilus), nor a combination of both, was found to be effective in
preventing infections women who had received a short course of antibiotic
treatment (Pirotta, BMJ 2004).
Theoretically, Lactobacillus bacteria may work, in part, by
helping to maintain a lower pH in the vagina, as maintaining a vaginal pH of
< 4.5 may help to reduce the risk of vaginal infections (Pendharkar, BMC Infect Dis 2015). A higher
vaginal pH (about 4.7 to 6) may be conducive to bacterial and/or yeast (Forsum, APMIS 2005; Galask, Am J Obstet Gynecol 1988).
Prostatitis
Prostatitis is an inflammation of the prostate gland. It is sometimes caused by
bacterial infection which can either be acute -- typically cured with
antibiotics, or chronic -- which is more difficult to treat and occurs more
frequently in men with gastrointestinal disturbances, such as irritable bowel
syndrome (IBS). A study among men in Italy with chronic prostatitis and IBS
found that taking a probiotic along with antibacterial treatment helped reduce
the risk of recurrent infection and the progression of inflammation to other
glands. At the beginning of the study all the men had antibiotic therapy which
temporarily eradicated bacterial infection in the prostate. For the next 12
months the men continued to receive antibiotic treatment (rifaxamin) for 7
consecutive days every month as well as one of the following every day 1) a
probiotic, 2) a placebo, 3) a probiotic for the first 6 months and then a
placebo for 6 months, 4) a placebo for 6 months and then a probiotic for 6
months. VSL#3 (now sold as Visbiome — see What to Consider When Buying for more
about this)(450 billion cells daily) was the probiotic used (see Results table below) as it has been shown to
reduce some (but not all) symptoms of IBS (discussed above). After 12 months, the
percentage of men found to have infections detected in a sperm sample was (by
treatment group) 13.6% (probiotic), 66.7% (placebo), 38.5% (probiotic/placebo),
and 56.5% (placebo/probiotic) (Vicari, Asian J Androl 2014).
Urinary tract infections (UTIs)
A review of nine randomized, controlled trials investigating the use of
probiotics of various strains and formulations for the prevention of UTIs
concluded that "no significant benefit was demonstrated for probiotics
compared with placebo or no treatment, but a benefit cannot be ruled out as the
data were few, and derived from small studies with poor methodological
reporting." (Schwenger, Cochrane Database Syst
Rev 2015).
One study that did find a reduction in UTIs involved 435 women with recurrent
UTIs and was conducted through medical centers in nine different countries
(including Germany, Hungary, Poland and Switzerland). The one-year study found
a 34% reduction in UTIs when a capsule containing 6 mg of harmless strains
of E. coli (Uro-Vaxom, OM PHARMA, Switzerland — not
currently available in the U.S.) was taken daily rather than a placebo (Bauer, Eur Urol 2005).
Mastitis
Certain probiotic strains have been found to be helpful in the treatment of
mastitis, a bacterial infection which can cause painful breast inflammation and
redness in nursing mothers, and a common cause of premature weaning. A study of
352 breastfeeding mothers with mastitis found that those who took a daily
probiotic (a capsule containing 90 billion cells of either Lactobacillus
fermentum CECT5716 or Lactobacillus salivarius CECT5713
— both being strains found in breast milk) for 21 days had significantly less
breast pain, lower total bacterial counts and greater rates of complete
recovery (88% and 85%, respectively) than women taking an antibiotic (29%) (Arroyo Clin Infect Dis 2010). Women taking
either strain of probiotic also had lower rates of recurrence of infection
(10.5% and 7.1%, respectively) compared to women who took an antibiotic
(30.7%).
Weight and fat loss
Several preliminary studies suggest that certain probiotics (alone or along
with a restricted diet) may help with fat loss or fat
redistribution. The effect of probiotics may be greater in women than in men.
However, better studies are required to determine if there is a real benefit,
and one study found increased weight with a probiotic.
In a preliminary study of overweight Japanese adults with
large amounts of abdominal fat, giving 100 billion cells of Lactobacillus
gasseri SBT2055 (LG2055) in a fermented milk product daily for 12
weeks significantly reduced abdominal visceral fat by 4.6% and abdominal
subcutaneous fat by 3.3% at the end of the study. Body mass index was also
significantly decreased by 1.5% and waist size decreased by 1.8% at the end of
the study (Kadooka, Eur J Clin Nutr 2010).
In a similar study using the same product, but giving lower doses of about 1.4
billion or 16 billion viable cells daily, abdominal visceral fat was
significantly reduced by about 8% with both doses after 12-weeks. Body mass
index was also reduced by 1.6% and 1.1%, respectively and waist circumference
reduced by 1.2% to 1.4% respectively at the end of the study. However,
subcutaneous visceral fat was not significantly reduced with these lower doses
(Kadooka, Br J Nutr 2013). In both studies, no
significant improvements occurred in the placebo group, which was given regular
fermented milk. (Note: The product used in these studies is not currently
available in the U.S. Several products in this Review, however, contain L.
gasseri, although the strain of L. gasseri may be
different from that in the study and the products contain additional
organisms.)
A study in Canada found a greater decrease in body weight
and fat mass among women taking a probiotic than among those taking placebo
during a 12-week diet (500 Calorie reduction in daily intake). Women taking the
probiotic lost an average 9.7 lbs, while those taking the placebo lost just 5.7
lbs. Women taking the probiotic continued to lose more weight (another 1.3 lbs)
during a subsequent 12-week period of normal caloric intake, but those taking
placebo had little further change in weight. The probiotic provided 162 million
cells (which, for a probiotic, is a relatively small dose) of Lactobacillus
rhamnosus CGMCC1.3724 (also called LPR) along with 300 mg of a
prebiotic mix of oligofructose and inulin per capsule. One capsule was taken 30
minutes before breakfast and another was taken 30 minutes before dinner. The
study included men, but those taking the probiotic lost no more weight than
those who received placebo, with both groups losing 9 to 10 lbs, on average,
during the calorie reduction phase and another 2 lbs over the subsequent 12
weeks. The researchers note that men tend to lose weight more easily than women
and the findings suggest that this probiotic formulation helps obese women
achieve sustainable weight loss (Sanchez, Br J Nutr 2013). The study was funded
by Nestle, which does not yet market this formula in North America.
A small, 12-week, placebo-controlled study in overweight
adults in Japan found that daily supplementation with a capsule containing 50
billion cells of Bifidobacterium breve B-3 resulted in a
slight (1.5 lb), but statistically significant, reduction in fat, although no
change in weight (apparently due to a simultaneous, small increase in muscle
mass). Improvements were also observed on certain measures of liver function
suggesting potential metabolic benefit (Minami, J Nutr Sci 2015).
(Several probiotics in this Review include B. breve, although not
specifically the B-3 strain).
Another study in Japan found that giving overweight and
obese men and women (ages 20 to 70 years old) 100 billion cells of the
probiotic strain Pediococcus pentosaceus LP28 which
had been heat-killed, for three months resulted in average decreases in
body fat mass of 2.6 lbs and in waist circumference of 1.1 inches compared to
placebo (Higashikawa, Eur J Clin Nutr 2016).
Interestingly, and contrary to the researchers' expectation, these beneficial
effects did not occur to the same degree among subgroup of participants given
live versions of the same probiotic. The researchers (some of whom have a
commercial interest in the probiotic) note that both the heat-killed and live
bacteria possess polysaccharides, which may play a role in the anti-obesity
effect. The probiotic used does not appear to be available in supplements sold
in the U.S.
Similarly, a placebo-controlled human trial in Spain found that 10 billion
cells of heat-killed Bifidobacterium animalis CECT 8145 (Ba8145)
taken daily for three months were modestly effective at reducing waist
circumference (by about ¾ of an inch) and various other markers of abdominal
obesity, including a very small reduction in BMI (down 0.35 from about 31). An
equivalent amount of a live version of the strain was less effective
than the heat-killed version and its effects were not statistically significant
compared to placebo. As with previous investigations, women benefited more than
men. Of note -- several of the scientists involved in this study were employees
of the company that owns the patent to Ba8145 (Pedret, Int J Obes 2019).
A small, 8-week study of overweight and obese women in Brazil
found that giving a probiotic along with a prescribed diet (providing only the
required amount of calories) resulted in greater reduction in waist
circumference than the diet alone (-5.5% vs -3.4%), but did not have a
significant effect on body weight or total body fat. Although the study
included a control (diet alone without probiotic) and was double-blinded, there
was no placebo treatment — a significant weakness of the study. The probiotic
treatment consisted of consuming, before breakfast, powder from packets
providing 5 billion cells of each of the following: Lactobacillus
acidophilus LA-14, Lactobacillus casei LC-11, Lactococcus
lactis LL-23, Bifidobacterium bifidum BB-06,
and Bifidobacterium lactis BL-4 (Gomes, Obesity 2017).
Increased weight with a probiotic was found
surprisingly found in a small study among obese adolescent Latino girls and
boys who maintained their typical diet and physical activity and took a sachet
of the probiotic (450 billion cells of an eight-strain combination of Lactobacillus, Bifidobacterium and Streptococcus known
as VSL#3 -- see What to Consider When Buying) mixed in a
non-caloric drink three times daily for 16 weeks. Among those who took the
probiotic, total and lower body fat increased by an average of 4.6 lbs. and 5
lbs., respectively, while those who took a placebo had an average decrease in
total and lower body fat of 2 lbs. and 0.4 lbs., respectively. There were no
significant effects on body mass index, liver fat, measures of insulin and
blood sugar, hormones that control appetite (such as leptin and ghrelin), or
the composition of bacteria in the gut (Jones, Pediatr Obes 2018).
Hypertension
Probiotics may modestly lower diastolic and systolic blood pressure, especially
when multiple strains are taken. A review of nine clinical trials found that
among 534 adults, those who consumed 100 billion colony-forming units of
probiotics (various strains from milk, cheese, yogurt or probiotic capsules)
daily for two months had a significant reduction in systolic blood pressure
(-3.56 mm Hg) and diastolic blood pressure (-2.38 mm Hg) compared to those who
did not consume probiotics. The effect was greater in those who consumed
multiple probiotic strains, and diastolic blood pressure was more improved in
those who had elevated blood pressure (>130/85) before the treatment (Khalesi, Hypertension 2014).
Blood pressure was not significantly improved in those who consumed fewer than
100 billion colony-forming units of probiotics per day, or who consumed
probiotics for less than two months.
Cholesterol-lowering
Probiotics (as capsules, yogurts or kefirs) may be helpful in modestly lowering
cholesterol, particularly by lowering LDL cholesterol. The evidence is mixed on
whether probiotics can help also lower triglycerides. Probiotics generally
don't seem to raise levels of HDL "good" cholesterol.
A study with Lactobacillus reuteri NCIMB 30242 (formerly sold
as Cardioviva -- now sold as UAS Lab's LRC) in men and women with high
cholesterol showed that those who took a capsule containing 2 billion cells
with breakfast and another with dinner for nine weeks had average reductions in
both LDL and total cholesterol of about 6% while these levels increased by a
few percent among people taking placebo. There was no effect on HDL cholesterol
nor triglycerides (Jones, Eur J Clin Nutr 2012).
A longer study (56 weeks) using a different probiotic, E. faecium M-74,
found that giving 2 billion cells once daily lead to a 12% reduction in total
cholesterol, with a 20% drop in LDL cholesterol -- although total cholesterol
and LDL levels also fell in the placebo group by 5.5% and 8.3%, respectively,
perhaps due to the fact that participants in both groups began eating healthier
during the study (Hlivak, Bratisl Lek Listy 2005). Lactobacillus
reuteri NCIMB 30242 may be the safer of the two probiotics as it is
generally recognized as safe by the FDA, while E. faecium has
not gained this status and some strains of this species are human pathogens (DiRienzo, Nutrition Reviews 2013).
LDL cholesterol and total cholesterol were also reduced
by 6.92% and 4.1% among adults with nonalcoholic fatty liver disease
(NAFLD) consuming a probiotic yogurt compared to those consuming the
same yogurt without probiotic organisms. NAFLD affects approximately 20 to 30%
of the population, is often associated with elevated cholesterol levels, and
can lead to cirrhosis of the liver. Among those taking the probiotic yogurt,
LDL and total cholesterol levels fell by a mean of 16 mg/dL and 11 mg/dL,
respectively, over an 8 week period, and body weight also decreased by nearly 4
lbs --- significantly more than in the group eating regular yogurt. As in other
studies with probiotics, there were no significant changes in levels of HDL
cholesterol or triglycerides, but there were significant improvements in levels
of liver enzymes ALT and AST, which are indicators of liver injury. Subjects
ate 300 grams (slightly more than 1 cup) per day of yogurt providing an average
of about 1.2 billion cells each of Lactobacillus acidophilus La5
and Bifidobacterium lactis Bb12 (Nabavi, J DairySci 2014).
Although other studies (above) have not shown
probiotics to lower triglyceride levels, a study in non-diabetic men and women
with borderline to moderately high triglyceride levels found that those who
took 2 grams of probiotic powder (Korea Yakult Co), providing 5 billion cells
each of Lactobacillus curvatus HY7601 and Lactobacillus
plantarum KY1032, daily for 3 months had a significant
reduction in triglyceride levels (about 18%) compared to those given a
placebo (Ahn, Atherosclerosis 2015). Also unusual is
that those taking the probiotic also had a significant increase in apo A-V
levels, a molecule that plays an important role in lipid metabolism and low
levels of which are associated with an increased risk of cardiovascular
disease.
Another study showing a reduction in triglycerides, as
well LDL levels, involved the probiotic L. fermentum ME-3.
People with borderline-high lipid profiles drank kefir (200 mL per day — a
little less than 1 cup) daily to which this probiotic (8 billion cells) had
been added. Over a period of 8 weeks, subjects experienced a 17% reduction in
triglycerides and reductions of 5% and 6%, respectively, in two forms of LDL --
LDL-C and ox-LDL. Those who drank the same kefir without the probiotic did not
experience these reductions (Mikelsaar, BMC Nutrition 2015).
Three of the researchers are owners of the patent on this probiotic.
Drinking kefir once daily for three months
significantly decreased triglycerides by about 25%, LDL-C cholesterol by 7.5%
and systolic and diastolic blood pressure by 9 mmHg and 7 mmHg, respectively,
in a study among 48 men and women in Brazil with metabolic syndrome.
These decreases were statistically significant compared to a placebo drink (a
non-fermented milk curd beverage), which did not improve any of these outcomes.
Women, but not men, who drank kefir also had modest increases in HDL
cholesterol that was significant compared to the placebo group. Male and female
participants consumed, respectively, 1.6 mL/kg and 1.9 mL/kg (i.e., about ½ cup
per day for a 175 lb. adult) of kefir or the placebo drink and were not
instructed to otherwise change their diet or exercise habits (Ghizi, PharmaNutrition 2021).
Cognitive function
A study among older adults showed that taking a probiotic daily for about three
months improved overall cognitive functioning by about 23% for those who were
cognitively impaired at baseline versus an 11% improvement among similarly
impaired people taking placebo. There was no significant improvement in
cognition among those with intact cognitive function. The probiotic contained
10 billion CFU of Lactobacillus rhamnosus GG (Culturelle
Vegetarian Capsules, which is similar to Culturelle Digestive
Health in this Review) from i-Health, which funded the study (Sanborn, Neuropsychiatr Dis Treat 2020).
Anxiety and depression
Chronic gut disorders are associated with higher rates of anxiety and
depression. This is has led to studies of probiotics in treating these
conditions. Although some preliminary studies have shown promising effects, the
overall results have been mixed.
A well-publicized study in mice suggested a possible role
for probiotics in reducing anxiety associated with gut inflammation (Bercik, Neurogastroenterol Motil 2011). Mice
in the study had a chemically-induced inflammation of their gut and exhibited
anxiety-like behavior. Giving 1 billion cells of Bifidobacterium longum NCC3001
for 1 week normalized their behavior. The probiotic did not reduce inflammation
in the gut but appeared to act by reducing the excitability of nerves in the
gut which connect, through the vagus nerve, with the central nervous
system. This same probiotic was given to men and women
with diarrhea-predominant or "mixed" IBS who also had
mild to moderate anxiety or depression. Clinically significant improvements in
depression occurred in twice as many people given the probiotic (14 of 22
patients) than among those given a placebo (7 of 22). However, here were no
significant improvements in anxiety, nor in physical symptoms of IBS. The
probiotic consisted of 10 billion cells of Bifidobacterium longum NCC3001
from a sachet dissolved in a drink once a day for six weeks (Pinto-Sanchez, Gastroenterology
2017). The study was funded by Nestle, which appears to be involved
in developing the probiotic. This probiotic is also known as BB536 from
Morinaga (Japan) and is found in various marketed products under that name.
Similarly, French researchers evaluated a combination of Bifidobacterium
longum R0175 and Lactobacillus helveticus R0052 (3
billion organisms total — sold in the U.S. and Canada by Jamieson as Probiotic Sticks with 1 billion cells per stick)
taken during or just after breakfast for 30 days in healthy individuals,
finding significant improvements in day-to-day depression, anger, anxiety, as
well as lower levels of the stress hormone cortisol in those taking the
probiotic compared to those taking placebo -- although some improvement also
occurred in the placebo group (Messaoudi, Br J Nutr 2011). In Holland, researchers found that giving healthy young
women and men a combination probiotic for 4 weeks reduced self-reported
negative thoughts in response to sad mood. This suggests that the probiotic may
help prevent depression. Specifically, the probiotic reduced thoughts that were
aggressive (wanting to hurt others or oneself) and ruminative (dwelling on
possible causes and consequences of one's distress). The probiotic, or a
placebo, was taken before bed as a powder mixed with water or lukewarm milk and
provided a total of 5 billion cells of two forms of Bifidobacterium (bifidum W23
and lactis W52) and five forms of Lactobacillus (acidophilus W37, brevis W63, casei 56, salivarius W24,
and lactis W19 and W58) supplied by Winclove (Amsterdam) —
although the company was not involved in the study or its funding (Steenbergen, Brain Behav Immun 2015).
A study in Malaysia among men and women (ages 18 to 60) who
reported having moderate levels of stress found that 1 billion cells of Lactobacillus
plantarum DR7 consumed once daily for three months reduced blood
levels of cortisol and inflammatory proteins (cytokines) compared to placebo,
although there were no overall reductions in symptoms of stress and anxiety,
nor improvements in symptoms of depression. Improvements were only seen on
selected measures in specific subgroups, such as modest decreases in stress and
anxiety among those younger than age 30 (Chong, Benef Microbes 2019).
A placebo-controlled study found that giving a prebiotic for 3
weeks (5.5 grams per day with breakfast) also reduced cortisol levels and
improved responses in one test associated with anxiety and depression
(measuring attention to positive and negative stimuli). These effects occurred
with the use of a galactooligosaccharide prebiotic (Bimuno®-GOS) but not
with a fructo-oligosaccharide prebiotic. However, neither prebiotic affected
other aspects of stress or anxiety, nor improved working memory and executive
functioning (Schmidt, Psychopharmacology 2014).
Consumption of fermented foods that contain probiotic
organisms may have a protective effect against symptoms of social anxiety,
according to a study of young adults. The study compared foods reported to have
been consumed to scores on personality tests. Those who most frequently
consumed the most fermented foods tended to also have lower levels of social
anxiety. This relationship was strongest in those who had higher levels of
neuroticism. Those who exercised most frequently tended to also have lower
levels of social anxiety. Fermented food items included yogurt, kefir,
fermented soy milk, miso soup, sauerkraut, some dark chocolates, microalgae
juices, pickles, tempeh and kimchi (Hilimire, Psychiatry Research 2015).
Autism spectrum disorder
Children with autism can experience significantly more gastrointestinal
symptoms, including diarrhea, constipation and abdominal pain, than others (McElhanon, Pediatrics 2014),
and probiotics may be helpful for some of these symptoms.
The constituents of the gut microbiota have been found to differ between people
with and without autism spectrum disorder (Kong, Nutrients 2019). As noted elsewhere, the makeup of the gut microbiota
may affect communication between the nervous system in the gut and the brain
(i.e., the gut-brain axis), which can influence behaviors and mood. In fact,
abnormal gut microbiota and gastrointestinal dysfunction in children with
autism has been associated with increased irritability, tantrums, aggressive
behavior and sleep disturbances (Critchfield, Gastroenterol Res Pract 2011).
One preliminary study in Taiwan among 71 boys with autism spectrum disorder
(age range 7 to 15) found that taking 30 billion cells of Lactobacillus
plantarum PS128 (Bened Biomedical Co.) per day for 4 weeks reduced
rule-breaking behavior and hyperactivity/impulsivity compared to baseline.
While these outcomes were not improved for those in the placebo group, it is
unclear if the between-group difference was significant (Liu, Nutrients 2019).
Another small study conducted in Boston among 27 people with
autism spectrum disorder (average age 10) found that taking the same probiotic
at twice the dose (60 billion cells per day) for 4 weeks in combination with
intranasal oxytocin (a prescription drug that has shown potential for improving
core symptoms of autism) significantly improved overall functioning, compared
to placebo plus oxytocin. The probiotic also tended to improve social and
behaviors measures, although this was not statistically significant compared to
placebo. Neither oxytocin nor the probiotic taken alone improved any of these
outcomes compared to placebo (Kong, Nutrients 2021).
One interesting, preliminary animal study found a specific bacterial
strain, Bacteroides fragilis, reduced gut permeability and
autism-like behaviors in mice (Hsiao, Cell 2013).
While these results are promising, larger and longer-term studies are needed
to confirm the benefit, if any, of probiotics for autism spectrum disorder
symptoms.
Mania
Probiotic supplementation was found to dramatically reduce rehospitalizations of
people who had been hospitalized due to acute mania in a small study (66
people) in Baltimore. After being discharged along with standard medication,
half the group was also given a probiotic tablet to take daily with a meal or
snack, while the other half was given an identical placebo tablet. Over 24
weeks, there were 8 psychiatric rehospitalizations in the probiotic group
versus 24 in the placebo group — a 74% reduction in the risk of
rehospitalization after adjusting for demographics. This reduction was even
higher (90%) among individuals who began the study with relatively high rates
of systematic inflammation. The study did not, however, find a difference in
levels of psychiatric symptoms during monthly visits, although these don't
necessarily capture the most severe symptom episodes. The probiotic used was
described as containing >100 million CFUs of Lactobacillus
(rhamnosus) GG and Bifidobacterium lactis strain BB1.
These strains are found independently or together in several products in the
Review below (e.g., USANA Probiotic), although the dose in the study was much
lower than the billions of CFUs typically in such products (ConsumerLab has
contacted the study's main author for clarification on the dose.) (Dickerson, Bipolar Disorders, 2018).
Allergy
A study in adults with grass pollen allergy who were taking a daily
antihistamine (loratadine 10 mg), found that adding a daily probiotic to the
regimen for 5 weeks resulted in a small, but statistically significant
reduction in the impact of allergy symptoms on the quality of their lives.
Without any medication, the subjects rated the impact of allergy symptoms on
their lives a 3.25 (mean score) on scale of 0 to 6, with 6 being extreme. This
fell to 1.65 with antihistamine treatment and to 1.40 when the probiotic was
added. Ocular symptoms improved with the added probiotic but nasal symptoms did
not (Costa, Eur J Clin Nutr 2014). The probiotic
consisted of a capsule with 2 billion cells of Lactobacillus
paracasei LP-33 which was taken with a meal. The study, which was
company-sponsored, included a later phase during which only the probiotic was
given, but results of this phase were not reported. Interestingly, a submission
to make an allergy health claim for this probiotic in Europe was retracted without explanation.
Somewhat similar to the findings above, a study in young adults with
self-reported seasonal allergy found that taking a probiotic for 8 weeks during
spring allergy season slightly reduced the impact of allergy symptoms on the
quality of life. Symptom scores (0 to 6, with 6 being extreme) were fairly low
to start: 1.71 in the probiotic group and 1.93 in the placebo group. At the
time of predicted peak pollen, scores fell slightly (by 0.68) in the probiotic
group and, surprisingly, also by a tiny amount (0.19) in the placebo group. The
probiotic was taken as a capsule twice daily and each capsule contained 1.2
billion cells of L. gasseri KS-13 and 150 million cells each
of B. bifidum G9-1 and B. longum MM-2. The
study was sponsored by the manufacturer, Wakunaga of America, and the USDA. (Dennis-Wall, Am J Clin Nutr 2017).
Childhood eczema/allergy:
The World Allergy Organization concluded there is a likely benefit in giving
probiotics to infants and pregnant or breastfeeding women to help prevent
eczema developing in children at high risk for developing allergy (Fiocchi, WAO Journal 2015).
Children at "high risk for developing allergy" are those with a
biological parent or sibling with existing or history of allergic rhinitis,
asthma, eczema, or food allergy — factors which raise the risk of developing
allergy to 20% to 30%. However, the organization did not find sufficient
evidence for probiotic use by these groups to reduce the risk of other types of
allergy in children. It also did not specify the probiotic type or dosage
regimen to be used, but a study in New Zealand
found this benefit with Lactobacillus rhamnosus HN001 (sold in
the U.S. as HOWARU® Rhamnosus). In the study (which also found
a reduction in gestational diabetes in
mothers), a dose of 6 billion cells daily of Lactobacillus rhamnosus HN001
or placebo was taken by mothers from week 35 of pregnancy to 6 months after
delivery, and infants took it from birth to age 2 years. By age 11, the
prevalence of eczema was 54% lower among children in the probiotic group than
among those in the placebo group, and the prevalence of hay fever was 27% lower
(Wickens, Pediatr Allergy Immunol 2018).
Atopic dermatitis (eczema):
Preliminary studies have shown that short-term supplementation with certain
species of probiotics may reduce symptoms of atopic dermatitis in both children
and adults when taken by mouth short-term. The effects of probiotics when
applied topically remains uncertain.
A study in Indonesia among 30 adults (average age 38)
with a history of mild to moderate atopic dermatitis found that taking a
probiotic daily for 8 weeks reduced the extent and severity of atopic
dermatitis (based on the SCORAD index) compared to placebo, although both
groups showed clinically meaningful improvement compared to baseline. SCORAD
index scores decreased by average of 72% among those in the probiotic group
compared to a 58% reduction among those given placebo. The probiotic provided a
daily dose of 20 billion cells of Lactobacillus plantarum IS-10506,
which was selected for its immunomodulatory effects (Prakoeswa, J Dermatolog Treat 2020). At this
time, it does not appear that any commercial products on the market in the U.S.
contain Lactobacillus plantarum IS-10506, a probiotic strain
isolated from dadih (a traditional fermented buffalo milk).
A daily probiotic given for 12 weeks was found to reduce symptoms of moderate
atopic dermatitis (recurrent, itchy, inflammation of the skin) in children
(ages 4 to 17 years) in Spain by 83% compared to 24% among those given placebo.
Children in both groups also received standard treatment of an oral
antihistamine, moisturizer, and, as needed, a topical steroid. Only 7.7% of the
probiotic-treated group required topical steroids to treat flare ups during the
study, compared to 10.8% of placebo group. The daily capsule contained 1
billion cells of an equal mixture of Bifidobacterium lactis CECT
8145, B. longum CECT 7347, and Lactobacillus casei CECT
9104, which were selected, respectively, for their antioxidant,
anti-inflammatory, and anti-pathogenic activities (Navarro-Lopez, JAMA Derm 2017).
A study of 20 children (ages 3 to 14 years) with mild to
moderate atopic dermatitis suggested that applying a spray containing Roseomonas
mucosa, a bacterium naturally found on the skin, to affected areas twice
weekly for 3 months, then every other day for an additional month, reduced the severity
of mild and moderate atopic dermatitis by at least 50% in 85% of children, with
benefits persisting for 8 months after treatment. However, there was no
comparison to placebo, so it is not possible to determine if the treatment was
effective. Each dose of solution contained up to 100 thousand cells of
live Roseomonas mucosa taken from healthy volunteers.
Researchers have postulated that Roseomonas mucosa may work by
reducing levels of Staphylococcus aureus, a bacterium that may
worsen this skin condition, and by increasing levels of certain skin oils that
tend to be lower in people with atopic dermatitis and that seem to improve skin
repair. There had been reports of infections among immunocompromised patients
associated with use of Roseomonas mucosa, but it was subsequently
determined that this species actually has low potential for causing infections
(Myles, Sci Transl Med 2020). At this time, it
does not appear that any commercial products on the market in the U.S.
contain Roseomonas mucosa.
Dandruff:
Taking a probiotic daily was shown to reduce signs and symptoms of dandruff in
a 56-day, well-controlled study among men in France. Although improvements were
noted in both the probiotic-treated and placebo groups within the first two
weeks of the study, by the end of the study improvements were significantly
greater in the probiotic group (70% reduction in free dandruff) compared to the
placebo group (23% reduction). Redness of the scalp decreased 58% in the
probiotic group vs. 31% in the placebo group. The probiotic consisted of 1
billion cells of Lactobacillus paracasei ST11, also known as
NCC 2461(Nestle), taken as a powder mixed into water (it is not currently sold
in North America). The researchers included employees of Nestle and L'Oreal. (Reygagne, Beneficial Microbes 2017).
Migraine:
A study among men and women (average age 38) with episodic or chronic migraine
found that 2 capsules of a blend of 14 bacterial strains (Bio-Kult by
Protexin) taken daily for 8 to 10 weeks reduced the frequency and severity, but
not the duration, of migraine attacks, compared to placebo. Among participants
with episodic migraine, those who took the probiotic had 2.6 fewer migraines
per month and a modest reduction in the severity of attacks, while migraine
frequency and severity remained relatively unchanged or slightly worse in the
placebo group. Participants with chronic migraine who took the probiotic had
9.7 fewer migraines per month and a modest reduction in the severity of
attacks. Medication use decreased among participants with both types of
migraine who took the probiotic, but remained about the same in those who took
the placebo. Each capsule of Bio-Kult contained 2 billion cells of the
following strains: Bacillus subtilis PXN 21, Bifidobacterium
bifidum PXN 23, Bifidobacterium breve PXN 25, Bifidobacterium
infantis PXN 27, Bifidobacterium longum PXN 30, Lactobacillus
acidophilus PXN 35, Lactob. delbrueckii ssp. bulgaricus PXN
39, Lactob. casei PXN 37, Lactob. plantarum PXN
47, Lactob. rhamnosus PXN 54, Lactob. helveticus PXN
45, Lactob. salivarius PXN 57, Lactococcus lactis ssp.
lactis PXN 63, and Streptococcus thermophilus PXN 66 (Martami, Cephalalgia 2019). (Note: Be aware
that fermented foods such as cheese and yogurt may trigger migraines in some people).
Preventing ADHD and Asperger syndrome in children
A small study in Finland used as the basis for the WHO probiotic recommendation
to prevent childhood eczema (above) also suggests probiotics may reduce the
risk of neuropsychiatric disorders. In the study, 10 billion cells of Lactobacillus
GG (Culturelle) were given daily to pregnant mothers for 4
weeks before expected delivery and then for 6 months after delivery to each
infant -- or the mother if breast-feeding. Follow up after 13 years found
that none of the 40 children who received the probiotic
developed attention deficit hyperactivity disorder (ADHD) or Asperger syndrome,
while among 35 who received placebo 6 children (17%) developed these
conditions: ADHD (3), Asperger syndrome (1), and both (2) (Partty, Ped Res 2015). Although statistically
significant, the researchers noted that the results should be considered
preliminary. They did not comment on the unusually high incidence in the
placebo group of Asperger syndrome, which is usually less than 1% but was 8.6%;
the percentage with ADHD was also slightly high.
Gestational (Pregnancy-related) Diabetes
Taking a probiotic during pregnancy may reduce women's risk of women developing
gestational (pregnancy-related) diabetes, according to a study in New Zealand.
In the study, pregnant women were given 6 billion cells of Lactobacillus
rhamnosus HN001 (also sold in the U.S. as HOWARU® Rhamnosus)
daily or placebo beginning in the third or fourth month of pregnancy and
continuing up to six months after giving birth. Among the women given the
placebo, 6.5% developed diabetes (defined in New Zealand as fasting blood sugar
≥ 99.1 mg/dL, or ≥ 162 mg/dL 2 hours after drinking glucose
solution), compared to only 2.1% of those given the probiotic. There were no
adverse effects on babies' size, weight or Apgar score (Wickens, Br J Nutr 2017).
Bone density and fractures
Animal studies suggest that bacteria in the gut may play a role in the
formation and maintenance of bone (Ohlsson, Trends Endocrinol Metab 2015; Li, J Clin Invest 2016). Probiotics can form
certain peptides during the fermentation process in the gut and these peptides
may benefit bone healing (Narva, Life Sci 2004; Parvaneh, Biomed Res Int 2015). Studies in
humans have shown modest benefits.
A study in Sweden among 68 older women (average age 76)
with normal bone density found that those who took 10 billion cells of L.
reuteri 6475 (provided by BioGaia AB, which funded the study) daily
for one year had smaller decreases in bone mineral density (BMD) of
the tibia (shin bone) than those who took a placebo (- 0.83% vs -1.85%),
although there were no significant differences between the groups in bone
mineral density loss in the hip and spine. The probiotic was given as a
freeze-dried powder in stick packs added to a cold drink or food, twice daily (Nilsson, J Intern Med 2018).
Two studies suggest that a specific probiotic strain may help to speed
the recovery of bone fractures, although it's not clear if there is a
long-term benefit. A study in 264 Chinese men and women (average age 65) with
fractured forearms found that taking a daily probiotic for three months,
beginning the day after the fracture occurred, significantly increased speed of
recovery (based on self-reported measures of hand function and pain) and range
of motion (as measured by a clinician) in the second and third months of
supplementation, compared to placebo. The study did not include imaging to
document the actual rate of fracture healing. The probiotic used was 12 billion
cells of Lactobacillus casei Shirota taken twice daily with
skim milk (sold in the U.S. as Yakult) (Zhang, J Orthop Surg Res 2019). A similar
study in China using a lower daily dose (6 billion cells twice daily) of the
same probiotic strain but similar outcome measures found that supplementation
increased the speed of recovery in the first four months, but by six months,
improvements were similar between those who took the probiotic and those who
took a placebo (Lei, Benef Microbes 2016).
Rheumatoid arthritis
Some research suggests that the gut microbiota in people with rheumatoid
arthritis differ from those of healthy people, and that these differences might
play a role in joint inflammation (Zhang, Nat Med 2015; Rogers, Nat Med 2015). There is also some
evidence that probiotic strains may reduce blood markers of inflammation in
people with rheumatoid arthritis (Pineda, Med Sci Monit 2011). However, a
clinical study in Brazil among 42 men and women (average age 58) with
rheumatoid arthritis found that taking 5 billion cells daily of a combination
of probiotic strains for two months in addition to standard medications (e.g.,
steroids such as prednisone or disease-modifying drugs such as methotrexate)
did not reduce the number of swollen and tender joints despite modest
reductions in certain blood markers of inflammation (IL-6 and TNF-a) compared
to standard medications plus placebo. The probiotic consisted of Lactobacillus
acidophilus LA-14, Lactobacillus casei LC-11, Lactococcus
lactis LL-23, Bifidobacterium lactis BL-04, and Bifidobacterium
bifidum BB-06 (Cannarella, Nutrition 2021).
Exercise performance
There is mixed evidence on whether or not probiotics improve exercise
performance. A small study among male runners found that taking 45 billion
cells daily of a combination of probiotic strains (Lactobacillus, Bifidobacterium and Streptococcus)
for one month increased endurance while running in a hot climate compared to
placebo (Shing, Eur J Appl Physiol 2014). However,
two placebo-controlled studies using Bacillis subtilis DE111
showed no benefit on performance: One was among 23 female college soccer
athletes taking 5 billion cells daily (along with a protein/carbohydrate
recovery drink) (Toohey, J Strength Cond Res 2018);
the other was among 25 male college baseball athletes who performed resistance
exercise 3 to 4 days per week and took one billion cells daily with a meal (or with
a whey protein/carbohydrate recovery drink immediately after exercise on
training days) for three months. The probiotic was provided by Deerland
Enzymes, which funded the study (Townsend, Sports (Basel) 2018).
Protein absorption
A study in 30 healthy, young adults found that taking a probiotic daily for two
weeks increased the absorption of amino acids from a milk protein supplement by
about 12% for arginine and 5% for isoleucine compared to taking milk protein
alone, although there was no significant difference in the absorption of other
amino acids. The daily probiotic was 1 billion cells of Bacillus
coagulans GBI-30, 6086 (GanedenBC30 by Kerry Inc., which funded the study),
which was given along with 25 grams of milk protein concentrate (ULTRANOR 9081
milk protein concentrate, Kerry Inc.) (Stecker, Nutr Metab 2020). GanedenBC30 is found in Schiff Digestive
Advantage Gas Defense Formula.
Other conditions
Although the evidence is not clear-cut, probiotics have been studied as a
treatment for many other conditions and their symptoms including: lactose
intolerance, respiratory and GI problems resulting from cystic
fibrosis, HIV-related diarrhea, cancer prevention, high
cholesterol, tuberculosis, eczema, acne, canker
sores, dental cavities, milk allergies, hay
fever, the prevention of respiratory infections in children,
and Crohn's disease. The American Gastroenterological
Association does not recommend the use of probiotics in Crohn's disease due to
insufficient evidence (Grace, Gastroenterology 2020).
See ConsumerTips™: What to Consider When Using for
dosage information.
Kombucha
Kombucha can be a good source of probiotics, although typically at about 1 billion
cells per cup -- an amount in the lower range of what is found supplements.
However, there are no clinical trials showing a benefit of kombucha in people.
In animals, kombucha has been shown to slow the digestion of carbohydrates,
lower blood sugar, reduce the absorption of cholesterol from the diet, and
decrease levels of certain liver enzymes (Aloulou, BMC Complement Altern Med 2012; Bellassoued, Pharm Biol 2015).
Animal studies also suggest kombucha may have protective effects on the liver (Murugesan, J Microbiol Biotechnol 2009): in
chickens, adding kombucha tea to chicken feed (instead of traditional
growth-promoting antibiotics) was shown to improve protein digestibility and
promote growth compared to standard feed (Afsharmanesh, Comp Clin Pathol 2014).
There is mixed evidence on whether or not kombucha contains glucuronic acid, a
compound that binds to and facilitates the elimination of certain drugs and
toxins in the body (Greenwalt LWT Food Sci Tech 1998; Greenwalt J Food Proct 2000).
The possible presence of glucuronic acid has been offered as an explanation of
liver-protecting effects of kombucha noted in animal studies (Murugesan, J Microbiol Biotechnol 2009). (At
least one kombucha product, GT's Kombucha Gingerade claims to
contain glucuronic acid (5 mg per cup) on the label.)
Fermented Dairy Foods
Fermented dairy foods, such as yogurts, may help with
certain forms of IBS and anxiety, as noted above, but have also been associated
with a lower risk of coronary heart disease (CHD). A study of
nearly 2,000 middle-aged men without CHD in Finland (where diary consumption is
high) found that those who, over the next 20 years, reported the highest intakes
of fermented dairy products had a 27% lower risk of developing
CHD than those with the lowest intakes. Furthermore, only consumption of
low-fat fermented dairy (i.e., less than 3.5% fat or, for yogurt, sour milk,
and buttermilk, less than 2.5% fat) was associated with a lower risk of CHD.
Most of the low-fat, fermented dairy consumed was sour milk (Koskinen, Br J Nutr 2018).
Interestingly, higher dairy intake, particularly from yogurt,
is also associated with a lower risk of developing high blood pressure in
middle-aged and older men and women. An analysis of results from two major
studies of health professionals -- including 20 to 30 years of follow-up --
found that at least five servings per week of yogurt versus less than one
serving per month was associated with a 16% lower risk of high blood pressure.
Furthermore, the combination of high yogurt intake with higher compliance with
the DASH diet was
associated with a 30% lower risk of high blood pressure (Buendia, J Hyperten 2018). Another
analysis of participants in the same studies found that increasing yogurt
consumption by at least 4 oz. per day was associated with a 11% lower risk of
developing type 2 diabetes over a 4-year period, compared to
maintaining the same consumption. The benefit was even greater if yogurt replaced
dairy foods higher in fat, such as cheese. The researchers found that
substituting 1 serving (8 oz) per day of yogurt for cheese was associated with
a 16% lower risk of developing type 2 diabetes; a slightly smaller reduction in
risk (12%) was found if substituting one serving of reduced-fat milk for cheese
(Drouin-Chartier, Am J Clin Nutr
2019).
Yogurt consumption has been associated with a
lower risk of colorectal cancer, although the benefit may be
stronger for men than for women. For example, an observational study of more
than 45,000 men and women in 10 different European countries found that, over a
12-year period, those who consumed the most yogurt (an average of about 3
ounces per day) had a 35% lower risk of being diagnosed with colorectal cancer
than those who did not consume yogurt, even after adjusting for calcium and
fiber intake and other known risk factors for colorectal cancer. The protective
effect was somewhat stronger in men than in women (Pala, Int J Cancer 2011). A later study that
analyzed data from more than 80,00 men and women found that, over a 26-year
period, men who consumed ≥ 2 servings of yogurt per week had a 19% lower
risk of colorectal polyps and a 26% lower risk of polyps with
a high potential to become malignant (turn into cancer) than men who did not
consume yogurt. The reduced risk was strongest for polyps occurring in the
colon rather than in the rectum, which the researchers speculated could be due
to the lower pH in the colon, which is "more hospitable for
probiotics." However, yogurt consumption was not associated
with a decreased risk of colorectal polyps in women (Zheng, Gut 2019). Yogurt
may help to prevent colon polyps by reducing inflammation and strengthening
the gut barrier, which may be of more benefit to men than to women because
men may be more vulnerable to some of the effects of impaired gut barrier
function than women (Yang, Cancer Epidemiol Biomarkers Prev 2016).
[Note: Preliminary research suggests that increased intake of dairy (possibly
due to casein) increases the risk of prostate cancer, as discussed in our Protein Powders Review.]
Summary of Evidence for Probiotics:
To help you chose an appropriate probiotic, the clinical importance of each
type of probiotic is summarized below. In general, Lactobacillus strains
have the widest range of applications, while Streptococcus strains
have the most limited positive evidence for diarrhea when combined with other
probiotics. Examples of products that contain certain probiotic organisms are
included in parentheses.
Uses of Probiotics in People — The Current Evidence (✓ = Effective; ½✓ = Partially Effective; ✗ = Not effective; ? = No
definitive results)
|
Abdominal Pain,
Bloating, & Consti- pation |
Diarrhea from
Antibiotics, Viral Infection, or Chemo-therapy |
Traveler's Diarrhea |
Weight Loss/ |
H. pylori Infection |
Colds and Respi-
ratory Infections |
Choles-terol-lowerers |
Anxiety/ |
Mouth and Throat
Infections |
Allergy |
Other Infections |
|
|
Bacillus |
✓ - In adults with and
without IBS - B. coagulans |
? - B.
coagulans |
|
|
|
|
? - B.
coagulans |
|
|
|
|
|
Bifido-bacterium |
In adults with IBS: |
✓ - B. lactis Bi-07
and B. lactis Bl-04 in combination with 2 strains of Lactobacillus (HOWARU
Restore) |
? |
✓ - B. breve B-3 |
✓- With Lacto-bacillus and Saccharo- |
✓ - B. lactis UABLA12
with L. acid- ophilus DDS-1 (UAS UP4 Junior) |
✓- B. lactis Bb12
with L. acid- ophilus La5 |
✓ - B. longum R0175
with Lacto- bacillus helveticus R0052; also other strains |
- |
- |
- |
|
Lacto-bacillus |
✓ In children - L. GG
(Culturelle) |
✓ In children - L. GG
(Culturelle) alone and with B. lactis Bb-12 and L.
acid- ophilus La-5; L. reuteri |
✓ - L. GG (Culturelle) |
✓ - L. gasseri SBT2055
(LG2055) |
✓ - Alone or with Bifido-bacterium and Saccharo- |
✓ - L. |
✓ - L. reuteri NCIMB 30242
(Cardio-viva™) and E. faecium M-74 (an Entero-coccus) |
✓ - L. helveticus R0052 with B.
longum R0175; also other strains |
✓ - For periodon- titis: L. reuteri |
½✓ - L. para-casei LP-33 with
anti- |
✓ - For vaginal infection: L. rhamnosus
GR-1 and L. fermentum RC-14 (Jarrow Fem- |
|
Strepto-coccus |
- |
✓ S. thermophilus with, L.
casei and, L. bulgaricus (Actimel) |
? |
- |
- |
- |
- |
- |
?- For throat infection: S. saliva-
rious K12 |
- |
- |
|
Saccha-romyces (yeast) |
✓ In adults with IBS - S. cerevisiae
CNCM I-3856 |
✓ S. boulardii (FloraStor) |
✓ S. boulardii (FloraStor) |
- |
✓ With Bifido-bacterium and |
- |
- |
- |
- |
- |
- |
For pets (small animals)
Studies in dogs and cats have shown that the addition of probiotics to the diet
can stimulate measures of immune function. For example, Enterococcus
faecium (SF68) has been shown to stimulate immune function in puppies
fed 500 million cells per day (Benyacoub 2003) and immune stimulation has
also been seen in cats fed 200 million cells per day of Lactobacillus
acidophilus DSM13241 (Marshall-Jones 2006). E. faecium,
at a dose of 2 billion cells daily, also reduced the incidence of prolonged
diarrhea in cats and dogs in an animal shelter (Bybee, J Vet Int Med, 2011),
but was not effective in treating giardiasis in dogs fed 500 million cells (Simpson, J Vet Int Med 2009).
Quality Concerns and
Tests Performed:
Neither
the FDA nor any other federal or state agency routinely tests probiotics for
quality prior to sale. However, quality issues for probiotic supplements can
include the following:
·
The viability of organisms in the product: How many organisms
are alive (in the case of active cultures) or can "come alive" from
their inactive and often freeze-dried state when purchased and used? Some
products make no claim at all and others only claim the amount at the time of
manufacture.
·
Lack of contaminating organisms: The product should contain the
bacteria and/or yeast strains that it claims on the label while potentially
pathogenic microorganisms and other microbial contaminants should not be
present. (This is of particular concern with enteric-coated products, as the
coating may protect contaminating pathogens from being naturally destroyed in
your stomach.)
·
Ability of pill to break apart properly: Tablets and caplets
which are not chewable must be able to properly break apart to release their
ingredients.
·
Protection of organisms from stomach: Some types of bacteria
cannot survive as they pass through stomach acid and into the small and large
intestines where the bacteria would grow (see ConsumerTips™: What to Consider When Buying for
more information). Ideally a product should contain bacteria that research
shows can survive passage through the stomach or, if its bacteria cannot
survive stomach acid, it should be enteric coated or use some other protective
formula (e.g., microencapsulation).
ConsumerLab.com, as part
of its mission to independently evaluate products that affect health, wellness,
and nutrition, purchased many leading probiotic products sold in the U.S. and tested
them to determine whether they 1) possessed the claimed amount of viable
bacteria listed on the label and at least 1 billion live organisms per
suggested daily adult serving (unless there is clinical evidence supporting a
lower dose), 2) were free of contamination from mold or types of bacteria with
disease-causing potential, and 3) disintegrated properly (if in tablet form) so
that their contents would be released, or if enteric-coated or delayed release,
their contents would be released, respectively, after passing through the
stomach. In addition, if product's ingredients include whole herb and/or 250 mg
or more of minerals daily, it must be found free of unacceptable levels of
lead, cadmium and arsenic. (See Testing Methods and Passing Score for
more information).
When purchasing products labeled as requiring refrigeration (not just after
opening), ConsumerLab.com had them shipped in refrigerated packaging and they
were maintained under refrigerated conditions throughout testing.
ConsumerLab.com tested all products prior their "best by" date.
What CL Found:
Among the probiotic supplements for people selected by CL for
testing, most passed testing but, as discussed below, a liquid probiotic was
discovered to be contaminated with a pathogenic bacteria, capsules of another
probiotic failed to live up to their claim of being enteric-coated, and a pet
product provided far fewer viable cells than promised.
·
Mary Ruth's Liquid Probiotic — Unflavored contained its
listed amount of probiotics but was contaminated with Pseudomonas
aeruginosa (36,900 CFU per 3 ml serving). Although there is no
established limit for Pseudomonas in foods or dietary
supplements in the U.S. or Europe, Pseudomonas can overgrow in
a liquid product eventually destroying the probiotic. Its presence is an
indication of poor quality control. According to the United States Pharmacopeia
(USP) a reasonable maximum bacterial Action Level for source water used in
pharmaceuticals is 500 CFU per mL. Mary Ruth's contained
nearly 25 times this limit. Pseudomonas is of particular concern to
immunocompromised individuals and infants (Eur Comm Health Cons Protect 2012).
·
NewRhythm Probiotics 50 Billion CFU contained
substantial levels of probiotics (81.4 billion which was more than the minimum
50 billion claimed at time of manufacture), however, it also claimed to be
enteric-coated but testing showed that this was not the case as it
disintegrated in simulated gastric fluid. Its label also did not disclose the
ingredients in the supposed enteric coating, which is required.
·
Rx Vitamins for Pets RxBiotic was found to
provide only 390 million CFU of probiotic organisms which is just 19.5% of its
claimed 2 billion CFU per scoop.
All of the other 20
products selected for testing by ConsumerLab provided the amounts of probiotics
listed on their labels and met the other requirements relating to quality. An
additional 15 products passed the same testing in CL's Quality Certification Program on
its label.
Interestingly, earlier tests of probiotics by ConsumerLab.com (2009 — 2015)
found a larger percentage products to not contain their claimed amounts of
viable cells, suggesting that many manufacturers have improved their products and/or
improved the conditions under which the products are shipped and stored prior
to purchase. This was also demonstrated in our review in 2018 when only one of
17 probiotics selected by CL for testing failed to contain its claimed number
of cells.
Range in CFUs
In addition to the variety of probiotic organisms included in probiotic
products (as seen in the listings in the second column of the Results table), there is great range in the
number of viable cells (or colony-forming units — CFUs) in suggested servings
of the products: We found as little as 100 million cells in Gerber
Soothe Probiotic Colic Drops and ProBiora Plus mints
(which, respectively, may be enough for infants and for oral care) to as much
as 225 billion in Visbiome High Potency Probiotic (which is
the original VSL#3 formula). In general, products provided
1 to 50 billion cells per serving, as shown in the graph below.
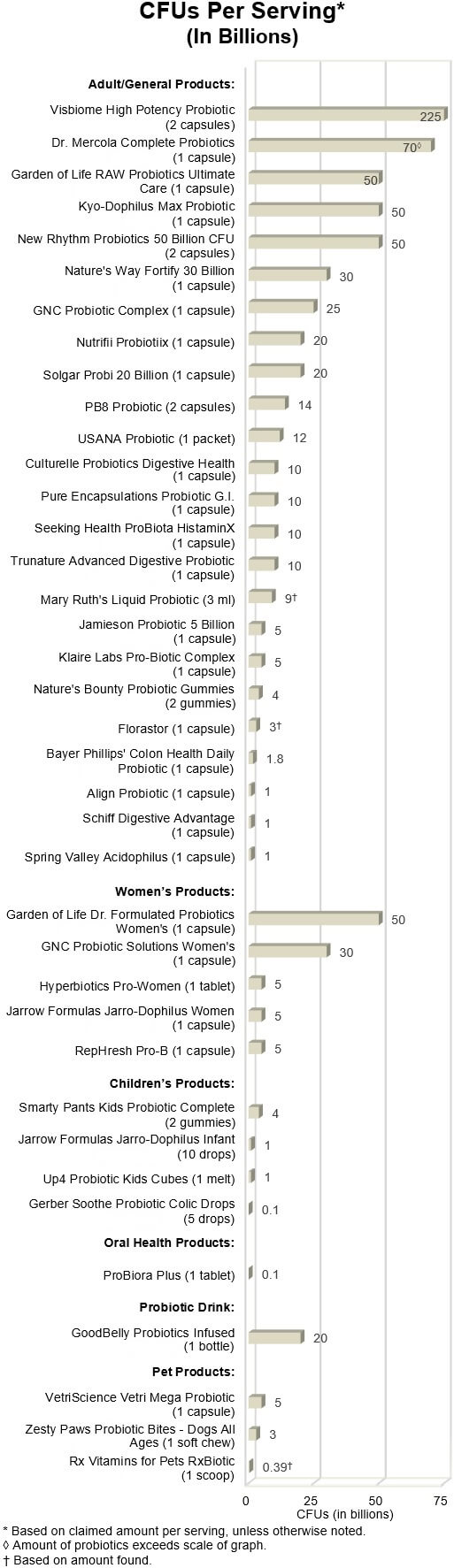
There was also variety in how products identified the amounts of organisms they
provided per serving. Some products clearly stated the amount in CFUs. Others
listed the CFU amount "at time of manufacture" of which some also
guaranteed a lower amount until expiration or the "Best By" date. A
few products only listed the weight of their probiotic ingredient and not a
specific number of cells.
If a product contained the amount claimed, it received a check in the second
column of the Results table below. If it did not, the
amount found is shown in the table. Per FDA regulations, if a product only
stated the amount "at time of manufacture," that amount was still
used as the expectation at time of use (so long as used prior to the expiration
or Best By date). Interestingly, all of the products that only showed amounts
at time of manufacture contained at least that amount and several contained
much more. For example, GoodBelly, Nature's Bounty
Probiotic Gummies, PB8, and SmartyPants Kids Probiotic Complete each
provided more than 4 times their listed amounts at time of manufacture; Spring
Valley Acidophilus provided twice the amount, and Hyperbiotics
Pro-Women delivered 16.3 billion cells, which was more than three
times its listed 5 billion cells at time of manufacture and more than 10
times its promised minimum of 1.5 billion cells at expiration.
Range in Cost Per Billion CFUs
The cost to get one billion viable probiotic cells from each product ranged
from just 1 cent for products containing the greatest number of cells (around
50 billion or more per serving) to $4 or more from those providing the smallest
number of cells (100 million). Otherwise, the most expensive source of
probiotic bacteria was Align at 65 cents per billion cells, as
shown in the graph below.
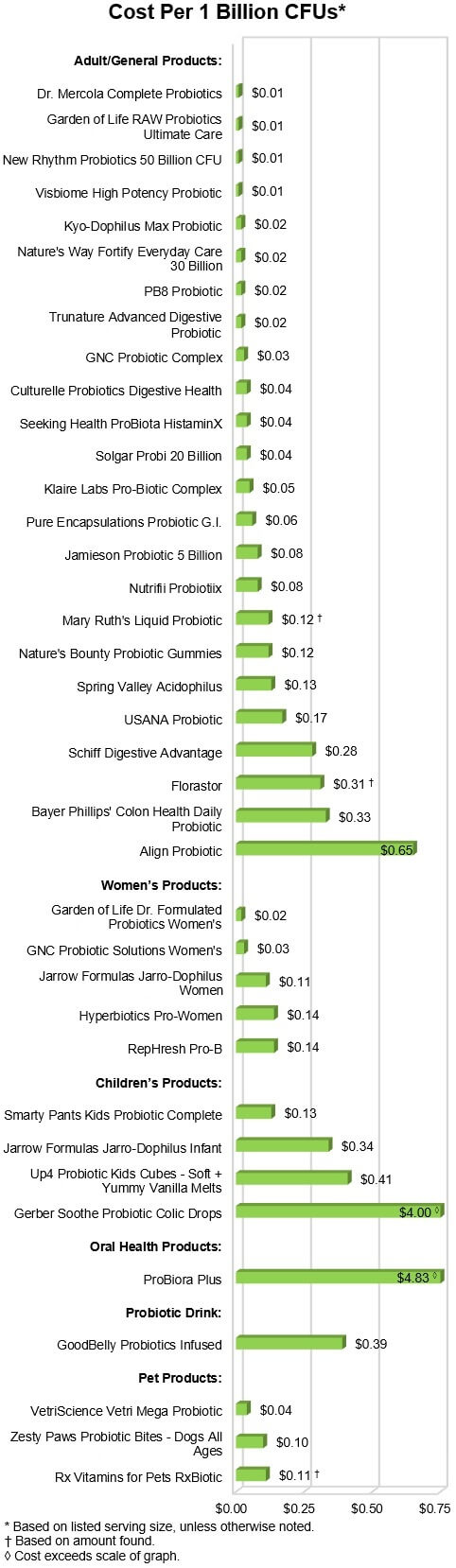
Top Picks:
It's
not possible to have one Top Pick among probiotics because the
desired strain(s) and dose depends upon the intended clinical use and there are
a wide variety of uses. It is best to consider what has been shown to work for
your condition (as discussed in the What They Do section above and the What to Consider When Using section
below) and then select an Approved product that most closely matches the
strain(s) and dose and is priced best. However, here are our Top Picks for
some common uses of probiotics:
·
Preventing antibiotic-associated diarrhea and traveler's
diarrhea: Culturelle Probiotics Digestive Health (37
cents per vegetarian capsule containing 10 billion cells) or, at much higher
cost, FloraStor ($1.94 for 2 vegetable capsules containing a
total of 6 billion cells). The strains in each of these products may help
prevent diarrhea from antibiotic use as well as traveler's diarrhea. Either
should be started the same day as when starting antibiotic treatment and
continued for one week after finishing the antibiotic. Take the probiotic at
least 2 hours after the antibiotic. For travel, start a few days before travel and
continue during travel.
·
Preventing C. difficile infection: FloraStor —
500 mg twice daily, should be started the same day as antibiotics and continued
for a week afterward. FloraStor contains S. boulardii,
which is among the strains recommended by the American Gastroenterological
Association for this use.
·
Treating constipation in adults, abdominal pain in children, and
colic in infants: Gerber Soothe Probiotic Colic Drops (containing Lactobacillus
reuteri DSM 17938). Although a relatively small amounts of cells, 5
drops (providing 100 million cells and costing 40 cents) given twice daily has
been shown to reduce constipation in adults and functional abdominal pain in
children. Given once daily to infants, it can reduce colic.
·
Reducing symptoms of irritable bowel syndrome (IBS): As noted earlier,
the American Gastroenterological Association does not recommend
the use of probiotics in treating IBS, having found supporting evidence to be
insufficient. With that said, Visbiome High Potency Probiotic ($1.67
per 2 capsules providing a total of 225 billion cells) may reduce bloating in
diarrhea-predominant IBS — although it may not improve other symptoms such as
abdominal pain, gas and urgency. About 4 capsules are taken twice daily.
(Note: Visbiome is the original VSL#3 formula, with which many
clinical trials were conducted. Due to legal disputes, what is now sold
as VSL#3 The Living Shield is not the original VSL#3 formula,
as explained in the What to Consider When Buying section). (Be
aware that there are concerns about taking the probiotic
currently sold as VSL#3 around the time of surgery.)
·
Pouchitis: Visbiome High Potency Probiotic — This
formulation has shown benefit when 300 billion cells, twice daily is taken and
the American Gastroenterological Association recommends this formulation.
·
Reducing flatulence: Schiff Digestive Advantage Intensive
Bowel Support provides 1 billion cells per capsule of Bacillus
coagulans GBI-30, 6086 (GanedenBC30). This dose was shown to modestly reduce the incidence of
flatulence (gas) in children compared to placebo when taken daily for three
months. Interestingly, this dose may also improve the absorption of certain amino acids from
milk proteins when taken with the milk protein (Stecker, Nutr Metab 2020). The Schiff
formula includes several digestive enzymes and costs 28 cents per
capsule — to be taken once daily with a meal.
·
Reducing symptoms of colds and respiratory infections: Many probiotics
make a claim of "immune support." This is a vague claim, although
there is some evidence that probiotics containing strains of Lactobacillus and/or Bifidobacterium may
modestly reduce symptoms of colds and upper respiratory infections, typically
when taken for 3 to 6 months around cold and flu season. Although products in
this review contain some of these organisms, none of the products have
themselves been tested. (An exception is that the 1 billion cells of GanedenBC30 in the Schiff Digestive
Advantage product modestly reduced the incidence, but not the
severity, of some cold symptoms in children).
We have no Top Pick for this category. It's worth noting that
a product tested in our 2018 review, UP4 Probiotics Children's,
provided a formula of 5 billion cells (1 billion cells of Lactobacillus
acidophilus DDS-1 and 4 billion cells of Bifidobacterium
lactis UABLA-12) that, if taken as soon as a household member is sick
and continued for two weeks, has been shown to reduce the severity of infection
in children. However, that product is no longer sold. The UP4
Probiotics Kids Cubes tested in the current review provides a total of
only 1 billion of these per suggested serving, so the potential benefit is
unknown.
·
Preventing vaginal infection: RepHresh Pro-B provides 5
billion cells per daily capsule (costing 70 cents) of a combination of L.
rhamnosus GR-1 and L. fermentum RC-14, and this
combination, at a daily dose of just 1 billion cells, has been shown to reduce
colonization of the vagina by potential pathogenic bacteria. This is not proof
that it will prevent infection, but it may help, making this product our Top
Pick in this category.
Pet probiotics
ConsumerLab.com selected three probiotic products for dogs and cats. One of
these, Rx Vitamins for Pets RxBiotic, contained only 19.5% of the 2
billion cells it claimed. VetriScience Vetri Mega Probiotic (for
dogs and cats) contained its 5 billion cells per capsule (18 cents) and Zesty
Paws Probiotic Bites (for dogs only) contained its 3 billion cells per
soft chew (29 cents). Both are oriented toward digestive health and contain
similar probiotic organisms, however, only Zesty Paws includes
Ganeden BC30 as its primary probiotic ingredient, which, as noted earlier,
reduced gas in children when taken for several months. On the other hand, VetriScience is
less expensive and provides more probiotic cells per dose than Zesty
Paws. We have no Top Pick in this category.
Kombucha
Although not tested in this review, ConsumerLab tested of kombucha products in
2018.The Top Pick for kombucha was GT's Kombucha ($2.22
per 8 fl oz cup in 2018, providing 1 billion cells). It is a true fermented
drink with a nice, effervescent, slightly acidic and gingery taste. In
addition, it has only 25 Calories per cup, which come from kiwi juice that gets
fermented in making the product.
Test Results by Product:
Listed
in the table below are the test results for 38 probiotic supplements -- 35
marketed for human use and three for use by pets. These are arranged by type
(adult/general, women's, children's, oral health, beverage and pet).
ConsumerLab.com selected 23 of the products. Fifteen other probiotics (each
indicated with a CL flask icon) were tested at the request of their
manufacturers/distributors through CL's voluntary Quality Certification Program and
are included for having passed testing.
Shown in the second column for each product are the labeled types of probiotic
organisms per suggested serving. If a product did not list an amount of viable
organisms or only stated the amount at time of manufacture, the amount found is
shown. Directions for use (including the suggested daily serving size) and
suggested storage conditions are shown in the third column. Cost comparisons
and pill sizes are shown in the fourth column, notable features in the fifth
column, and the full list of ingredients is provided in the last column.
Results
of ConsumerLab.com Testing of PROBIOTIC SUPPLEMENTS
(Click arrows or swipe left or right to see all columns)
Approval Status
Product Name
(Suggested Serving on Label)
Claimed Amount (in CFUs) and Type of Probiotic
Organisms in Suggested Servingⓘ
Microbial Contamination
Listed Storage Conditions
Directions
Cost per Serving
[Cost per 1 Billion Organisms]
Price
Pill Sizeⓘ
Notable Features
Full List of Ingredients Per Serving
Adult/General Products:
APPROVED
Align® Probiotic
Dist. by Procter & Gamble
1 capsule
1 billion
(At manufacture, 10 million until "best by" dateⓘ)
✔
Found 1.1 billion
Bifidobacterium longum subsp. longum 35624™
Microbial Contamination: Pass
For best results, we recommend you store Align below 86° F (30° C) in the
original blister unit. No Refrigeration Required.
Take one capsule per day.
$0.65/capsule
[$0.65]
$41.24/63 capsules
Medium/large capsule
Gluten free. Soy free. Vegetarian.
Precaution: Contains: milk.
1 capsule
Bifidobacterium longum subsp. longum 35624™ 4 mg.
Other Ingredients: Microcrystalline cellulose, hypromellose, sucrose, magnesium
stearate, sodium caseinate, more...
APPROVED
Bayer Phillips'® Colon Health® Daily Probiotic
Dist. by Bayer HealthCare LLC
1 capsule
1.75 billion
(When manufactured and provides a level of probiotic of 1.5 billion CFUs at
time of expirationⓘ)
✔
Found 1.3 billion
Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, Bifidobacterium
longum MM-2
Microbial Contamination: Pass
Store at room temperature.
Adults: Take one capsule daily with a meal.
$0.58/capsule
[$0.33]
$25.99/45 capsules
Medium/large capsule
This product does not contain gluten.
1 capsule
Proprietary Blend (Lactobacillus gasseri KS-13, Bifidobacterium
bifidum G9-1 and Bifidobacterium longum MM-2) 1.75
billion CFUs.
Other Ingredients: Potato Starch, Gelatin, Silicon Dioxide
APPROVED
Top Pick
for preventing antibiotic-associated diarrhea and traveler's
diarrhea
Culturelle® Probiotics Digestive Health
Dist. by i-Health, Inc.
1 vegetarian capsule
10 billion
✔
Lactobacillus rhamnosus GG
Microbial Contamination: Pass
Store Culturelle® in a cool, dry place away from direct sunlight.
Directions for 12 years of age and older: For daily use, take one (1) capsule
per day. For optimal results, continued daily use is suggested. If experiencing
occasional digestive upset, take two (2) capsules daily [one (1) in the morning
and one (1) at night] until discomfort subsides, more...
$0.37/capsule
[$0.04]
$22.49/60 vegetarian capsules
Medium/large vegetarian capsule
1 vegetarian capsue
Inulin 200 mg
Contains none of the following: artificial dyes, preservatives, dairy,
lactose, milk, yeast, wheat, sugar, gluten or soy.
Precaution: While there are no dairy-derived ingredients in the
product, it is produced in a facility that also handles dairy ingredients.
1 vegetarian capsule
Calories 0, Total Carbohydrate 0 g, Total Sugars [Incl. 0 g Added Sugars] 0
g, Lactobacillus rhamnosus GG 10 billion CFUs, Inulin (Chicory
Root Extract) 200 mg.
Other Ingredients: Hydroxypropyl methylcellulose, sucrose, maltodextrin, sodium
ascorbate, vegetable magnesium stearate, silicon dioxide, and titanium dioxide
(color).
APPROVED
Dr. Mercola® Complete Probiotics
Dist. by NHP
1 capsule
70 billion
✔
Lactobacillus acidophilus DDS®-1, Bifidobacterium lactis,
Lactobacillus plantarum, Lactobacillus casei, Lactobacillus rhamnosus,
Lactobacillus brevis, Bifidobacterium longum, Lactobacillus salivarius,
Streptococcus thermophilus, Bifidobacterium bifidum
Microbial Contamination: Pass
Refrigeration not required.
Adults, as a dietary supplement, take one (1) capsule daily, preferably in the
morning.
$1.00/capsule
[$0.01]
$29.97/30 capsules
Large capsule
Delayed release.
1 capsule
Dr. Mercola's Special Blend [Lactobacillus acidophilus DDS®-1, Bifidobacterium
lactis, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus rhamnosus,
Lactobacillus brevis, Bifidobacterium longum, Lactobacillus salivarius,
Streptococcus thermophilus, Bifidobacterium bifidum) 270 mg, more...
APPROVED
Top Pick
for preventing antibiotic-associated diarrhea, traveler's
diarrhea, and C. difficile infection
Florastor®
Mfd. by Biocodex
1 vegetable capsule
250 mg
✔
Found: 3 billion
Saccharomyces boulardii CNCM I-745
Microbial Contamination: Pass
Do not refrigerate.
2 capsules 1-2 times daily.
$0.97/capsule
[$0.31 based on amount found]
$28.99/30 vegetable capsules
Large vegetable capsule
1 vegetable capsule
Lactose 32.5 mg
1 vegetable capsule
Saccharomyces boulardii CNCM I-745 250 mg, Lactose 32.5 mg.
Other Ingredients: Magnesium stearate, hydroxypropyl-
methylcellulose.
APPROVED
Garden of Life® RAW Probiotics Ultimate Care![]()
Dist. by Garden of Life LLC
1 vegetarian capsule
100 billion
(At expiration date under recommended storage conditions)
✔
ISS Bif™ Bifidobacterium lactis (SD-5219), Lactobacillus
paracasei (SD-5218), Bifidobacterium lactis (SD-5220), Bifidobacterium
lactis, Bifidobacterium longum, more...
Microbial Contamination: Pass
Heavy metalsⓘ:
Pass
Best if refrigerated. Store in a dry place 71° F (22° C) or below.
Adults take 1 capsule daily.
$1.28/capsule
[$0.01]
$38.49/30 vegetarian capsules
Medium/large vegetarian capsule
1 vegetarian capsule
Protein-digesting enzyme blend 50 mg, Eastern European RAW fruit and veggie
blend 45 mg
Contains no binders, fillers, carries, artificial colors or preservatives.
RAW. Gluten free. Soy free. Non GMO.
1 vegetarian capsule
Replenish Blend [ISS Bif™ Bifidobacterium lactis (SD-5219), Lactobacillus
paracasei (SD-5218), Bifidobacterium lactis (SD-5220),
RAW Whole Food Probiotic Blend: Bulgarian Yogurt (milk) Concentrate, Eastern
European Wild Kefir Culture containing Bifidobacterium lactis,
Bifidobacterium longum, Lactobacillus acidophilus, more...
APPROVED
Dist. by General Nutrition Corporation
1 vegetarian capsule
25 billion
✔
Lactobacillus acidophilus (CUL 60), Lactobacillus
acidophilus (CUL 21), Bifidobacterium bifidum (CUL
20), Bifidobacterium animalis subsp. lactis (CUL
34)
Microbial Contamination: Pass
No refrigeration required.
Take one capsule daily with food. One to two additional servings may be taken
daily as desired for added support.
$0.75/capsule
[$0.03]
$44.99/60 vegetarian capsules
Large vegetarian capsule
1 vegetarian capsule
Fructooligo-
saccharides (FOS) 100 mg.
Gluten free. Lactose free.
1 vegetarian capsule
LAB4 Probiotics [Lactobacillus acidophilus (CUL 60), Lactobacillus
acidophilus (CUL 21), Bifidobacterium bifidum (CUL
20), Bifidobacterium animalis subsp. lactis (CUL
34)] 25 Billion Active Cultures, Fructooligo-
saccharides (FOS) 100 mg, more...
APPROVED
Dist. by Jamieson Laboratories
1 capsule
5 billion
(Guaranteed to expiry)
✔
Lactococcus lactis (UALI-08), Lactobacillus gasseri (UALg-05), Lactobacillus
rhamnosus (UALr-06), Bifidobacterium animalis subsp. lactis, Bifidobacterium
breve (UABbr-11)
Microbial Contamination: Pass
Store between 15° C and 25° C.
Adults, adolescents, and children 6 years and over: Take 1 capsule daily with a
meal. Children 3-5 years (under parent's supervision): Empty powder/contents of
1 capsule daily directly into food, yogurt, etc. and mix until dissolved.
$0.42/capsule
[$0.08]
$29.99/72 capsules
Medium/large capsule
Non-GMO. No gluten, lactose, artificial flavours
or preservatives. No Animal Testing. Vegetarian.
1 capsule
Probiotic Complex [Lactococcus lactis (UALI-08) 3.5 billion
CFU, Lactobacillus gasseri (UALg-05) 0.5 billion CFU, Lactobacillus
rhamnosus (UALr-06) 0.5 billion CFU, Bifidobacterium animalis subsp. lactis 0.4
billion CFU, Bifidobacterium breve (UABbr-11) 0.1 billion CFU]
5 Billion CFU, more...
APPROVED
Klaire Labs™ Pro-Biotic Complex™
Mfd. by SFI USA
1 vegetarian capsule
5 billion
✔
Lactobacillus acidophilus, Bifidobacterium bifidum
Microbial Contamination: Pass
Keep refrigerated.
1 capsule, 1 or 2 times daily with food or as directed by a healthcare
professional.
$0.24/capsule
[$0.05]
$24.19/100 vegetarian capsules
Large vegetarian capsule
Free of the following common allergens:
milk/casein, eggs, fish, shellfish, tree nuts, peanuts, wheat, gluten, and
soybean. Contains no artificial colors, flavors, or preservatives.
1 vegetarian capsule
Probiotic Blend (5 billion CFU) in a base of inulin (derived from chicory root)
[Lactobacillus acidophilus 2.5 billion CFU, Bifidobacterium
bifidum 2.5 billion CFU] 433 mg.
Other Ingredients: Vegetarian capsule (hydroxypropyl methylcellulose, water)
and ascorbyl palmitate.
APPROVED
Mfd. by Wakunaga of America Co., LTD.
1 vegetarian capsule
50 billion
(Guaranteed live at expiry)
✔
Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, Bifidobacterium
longum MM-2, Lactobacillus plantarum UALp-05, Lactobacillus
paracasei UALpc-04, Bifidobacterium lactis, more...
Microbial Contamination: Pass
Refrigeration not required.
Take one capsule daily. Not recommended for children under 12.
$1.00/ capsule
[$0.02]
$29.97/30 vegetarian capsules
Large vegetarian capsule
Free of: GMOs, soy, gluten, dairy, sodium,
preservatives, artificial colors and flavors.
1 vegetarian capsule
Proprietary Blend [Lactobacillus gasseri KS-13, Bifidobacterium
bifidum G9-1, Bifidobacterium longum MM-2, Lactobacillus
plantarum UALp-05, Lactobacillus paracasei UALpc-04, Bifidobacterium
lactis UALBla-01, Bifidobacterium longum BB536, more...
NOT APPROVED
Mary Ruth's® Liquid Probiotic - Unflavored
Dist. by MaryRuth Organic LLC
3 ml
3000 mg
✔
Found: 9 billion
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus
salivarius, Lactobacillus casei, Lactobacillus plantarum, Lactococcus lactis,
Streptococcus themophilus, Bifidobacterium bifidum, more...
Microbial Contamination: Failed: Found Pseudomonas
aeruginosa (36,900 CFU)
No Refrigeration Necessary.
First time use: Take 1/2 dose, preferably with food or liquids. Gradually
increase dose as you see fit. Adults: Take 3 ml (60 drops, 4 pumps or 16
sprays) with water, juice, or alone, more...
$1.12/3 ml
[$0.12 based on amount found]
$44.95/4 fl oz [120 ml] bottle (approx. 40 servings)
Liquid from bottle
Non GMO. Vegan. Gluten free, Corn Free, Nut
Free, Soy Free. CCOF Certified organic seal. USDA organic seal.
3 ml
Proprietary Blend [Lactobacillus acidophilus, L. rhamnosus, L. salivarius,
L. casei, L. plantarum, Lactococcus lactis, Streptococcus thermophilus,
Bifidobacterium bifidum, B. lactis, B. infantis, B. breve, B. longum] 3,000
mg.
Other Ingredients: A Blend Of Probiotics In An Enzyme-Enriched Substrate, more...
APPROVED
Nature's Bounty® Probiotic Gummies - Pineapple,
Raspberry & Orange Flavored Gummies![]()
Dist. by Nature's Bounty, Inc.
2 gummies
Over 4 billion
(At the time of manufactureⓘ)
✔
Found 17.2 billion
Bacillus coagulans Unique IS-2™
Microbial Contamination: Pass
No storage conditions listed.
For adults, enjoy two (2) gummies daily.
$0.50/2 gummies
[$0.12]
$29.95/two bottle of 60 gummies (120 gummies total)
Medium/large gumdrop-shaped gummy
Non-GMO. No artificial flavor, no artificial
sweetener, no preservatives, no milk, no lactose, no soy, no gluten, no wheat,
no yeast, no fish.
2 gummies
Calories 20, Total Carbohydrate 4 g, Total Sugars [Includes 2 g Added Sugars] 2
g, Vitamin D (as Cholecalciferol) 10 mcg (400 IU), Sodium 15 mg, Bacillus
coagulans Unique IS-2™ contains over 4 billion live cultures at the
time of manufacture.
Other Ingredients: Glucose Syrup, Sugar, Pineapple Juice Concentrate, more...
APPROVED
Nature's Way Fortify™ Everyday Care 30 Billionⓘ![]()
Dist. by Nature's Way Brands, LLC
1 veg capsule
30 billion
(Until the date of expiration without refrigeration)
✔
Lactobacillus paracasei Lpc-37®, Lactobacillus plantarum Lp-115®, Lactobacillus
rhamnosus GG™, Lactobacillus acidophilus La-14®, Lactobacillus
rhamnosus HN001™, Lactobacillus brevis Lbr-35™, Lactobacillus
casei Lc-11®, Bifidobacterium lactis Bi-07®, Bifidobacterium
lactis/bifidum Bb-02™, Bifidobacterium lactis Bl-04®, Bifidobacterium
lactis HN019™
Microbial Contamination: Pass
Store in dry place at 73° F (23° C) or below. May be refrigerated.
Take 1 capsule daily.
$0.73/veg capsule
[$0.02]
$21.99/30 veg capsules
Large veg capsule
Delayed-release. Non GMO Project Verified seal.
No Artificial Colors. Gluten Free.
1 veg capsule
Fortify™ Daily 30 Billion CFU [Proprietary Probiotic Blend: Lactobacilli Blend
(22 Billion CFU) [Lactobacillus paracasei Lpc-37®, Lactobacillus
plantarum Lp-115®, Lactobacillus rhamnosus GG™, Lactobacillus
acidophilus La-14®, Lactobacillus rhamnosus HN001™, Lactobacillus
brevis Lbr-35™, Lactobacillus casei Lc-11®], Bifidobacteia Blend
(8 Billion CFU) [Bifidobacterium lactis Bi-07®, Bifidobacterium
lactis/bifidum Bb-02™, Bifidobacterium lactis Bl-04®, Bifidobacterium
lactis HN019™] 550 mg, Chicory Root Fiber 50 mg.
Other Ingredients: Cellulose, plant-derived capsule (hypromellose, gellan gum),
magnesium stearate, silica.
NOT APPROVED
NewRhythm Probiotics 50 Billion CFU
Dist. by New Rhythm
Note: Label
violation
Product does not disclose ingredients in its enteric coating
2 vegetarian capsules
50 billion
(Contains a minimum of 50 billion live bacteria when manufactured, and provides
an effective level until at least the "best by" dateⓘ)
✔
Found 81.4 billion
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus crispatus,
Lactobacillus plantarum, Lactobacillus paracasei, more...
Microbial Contamination: Pass
Enteric coated disintegration: Failed. Fully disintegrated in gastric fluid,
indicating enteric-coating was not effective.
Shelf stable. No refrigeration required.
Take two (2) veggie capsules daily or as directed by a healthcare professional.
$0.57/2 capsules
[$0.01]
$16.99/60 vegetarian capsules
Large vegetarian capsule
2 vegetarian capsules
Organic prebiotic fiber blend 150 mg
Verified non-GMO. Gluten free. Target release. Vegetarian. Does not contain:
gluten, soy, corn, lactose, egg, fish, shellfish, milk, tree nuts, rice, sugar,
wheat, yeast, starch, peanuts, fillers, binders, sulfates, sodium, artificial
ingredients, colors, flavors, or magnesium stearate.
2 vegetarian capsules
Proprietary Probiotic Blend [L. acidophilus, L. rhamnosus, L. crispatus, L.
plantarum, L. paracasei, L. bulgaricus, L. reuteri, L. casei, L. salivarius, L.
helveticus, L. gasseri, B. lactis, Pediococcus pentosaceus, B. breve, B.
adolescentis, B. infantis, B. longum, Lactococcus lactis, Pediococcus
acidilactici, Streptococcus thermophiles] 50 billion CFU, Organic Prebiotic
Fiber Blend 150 mg, more...
APPROVED
Nutrifii™ Probiotiix™ⓘ![]()
Dist. by Ariix
1 vegetable capsule
20 billion
✔
Lactobacillus gasseri, Lactobacillus plantarum, Bacillus subtilis,
Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium longum,
Lactobacillus casei, Lactobacillus paracasei, Lactobacillus rhamnosus,
Lactobacillus reuteri, Lactobacillus salivarius, Streptococcus thermophilus,
Lactobacillus fermentum, Saccharomyces boulardii, Lactobacillus acidophilus
Microbial Contamination: Pass
Store in a cool, dry place, protected from light.
Take 1 capsule per day, 1-2 hours before or after eating. When used in
conjunction with antibiotics: Take the probiotic 1-2 hours after antibiotic
ingestion and 3-7 days beyond antibiotic completion.
$1.63/vegetable capsule
[$0.08]
$45.69/28 vegetable capsule
Very large vegetable capsule
1 vegetable capsule
Prebiotic inulin 250 mg
Soy Free. Gluten Free. Non-GMO. Dairy Free. 100% Vegan.
1 vegetable capsule
Probiotic Blend (Lactobacillus gasseri, Lactobacillus plantarum, Bacillus
subtilis, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium
longum, Lactobacillus casei, Lactobacillus paracasei, Lactobacillus rhamnosus,
Lactobacillus reuteri, Lactobacillus salivarius, Streptococcus thermophilus,
Lactobacillus fermentum, , more...
APPROVED
PB8™ Probiotic
Dist. by Church & Dwight Co., Inc.
2 capsules
14 billion
(At time of manufactureⓘ)
✔
Found 59.6 billion
Lactobacillus acidophilus LA-14®, Bifidobacterium lactis BL-04®, Lactobacillus
plantarum LP-115®, more...
Microbial Contamination: Pass
Store in cool, dry place; best if refrigerated after opening.
As a dietary supplement, adults take 2 capsules per day.
$0.63/2 capsules
[$0.02]
$37.99/120 capsules
Large capsule
This product contains no gluten, eggs, milk,
peanuts, tree nuts, or soy.
2 capsules
Total Carbohydrate <1 g, Proprietary Probiotic Blend [14 billion CFU]
[Microcrystalline cellulose, Lactobacillus acidophilus LA-14®, Bifidobacterium
lactis BL-04®, Lactobacillus plantarum LP-115®,
Magnesium stearate, Silicon dioxide, more...
APPROVED
Pure Encapsulations® Probiotic G.I.![]()
Dist. by Pure Encapsulations
1 capsule
10 billion
✔
Bifidobacterium lactis (Bl-04), Lactobacillus acidophilus (La-14), Lactobacillus
salivarius (Ls-33), Lactobacillus casei (Lc-11), Streptococcus
thermophilus (St-21), Bifidobacterium bifidum (Bb-06)
Microbial Contamination: Pass
Shelf-stable.
Take 1 capsule, 1-2 times daily, with or between meals.
$0.61/capsule
[$0.06]
$36.70/60 capsules
Large capsule
Gluten-free, Dairy-free, Soy-free. Non-GMO &
hypoallergenic.
1 capsule
Probiotic blend [Bifidobacterium lactis (Bl-04), Lactobacillus
acidophilus (La-14), Lactobacillus salivarius (Ls-33), Lactobacillus
casei (Lc-11), Streptococcus thermophilus (St-21), Bifidobacterium
bifidum (Bb-06)] 10 billion CFU, more...
APPROVED
Top Pick
for reducing flatulence
Schiff® Digestive Advantage®
Dist. by Reckitt Benckiser
1 capsule
1 billion
✔
BC30 Bacillus coagulans GBI-30,
6086
Microbial Contamination: Pass
Store in a cool, dry place. No refrigeration necessary.
Take one (1) capsule daily with a meal. Some individuals may benefit from
taking two or more capsule daily. Do not take more than three (3) capsules
daily.
$0.28/capsule
[$0.28]
$26.99/96 capsules
Medium/large capsule
Guaranteed: No added sugar (sucrose, fructose,
lactose), salt (sodium chloride) or yeast. No preservatives or artificial
flavors. Guaranteed for purity, freshness and labeled potency.
Precaution: Contains wheat and soy.
1 capsule
BC30 Bacillus coagulans GBI-30,
6086 1 billion viable cells, Protease (from Aspergillus oryzae)
30,000 HUT, Amylase (from Aspergillus oryzae) 450 DU, Lipase
(from Rhizopus oryzae) 30 FIP.
Other Ingredients: Maltodextrin, hypromellose, more...
APPROVED
Seeking Health® ProBiota HistaminXⓘ![]()
Dist. by Seeking Health, LLC
1 acid-resistant vegetarian capsule
10 billion
(At the time of manufactureⓘ)
✔
Bifidobacterium infantis, Bifidobacterium bifidum, Bifidobacterium longum,
Lactobacillus salivarius, Lactobacillus plantarum, Bifidobacterium lactis,
Bifidobacterium breve
Microbial Contamination: Pass
Enteric coated disintegration: Pass
Refrigerate and keep tightly closed to maintain maximum potency.
Take one capsule any time of day with or without food or as directed by your
healthcare professional.
$0.42/capsule
[$0.04]
$25.46/60 acid-resistant vegetarian capsule
Does Not Contain: Milk, eggs, fish, shellfish, tree
nuts, peanuts, wheat, or soy.
1 acid-resistant vegetarian capsule
Probiotic Blend [(Bifidobacterium infantis, Bifidobacterium bifidum,
Bifidobacterium longum, Lactobacillus salivarius, Lactobacillus plantarum,
Bifidobacterium lactis, Bifidobacterium breve) Total cultures 10 Billion CFU]
77 mg, more...
APPROVED
Dist. by Solgar, Inc.
1 vegetable capsule
20 billion
✔
Lactobacillus plantarum LP229v™
Microbial Contamination: Pass
Shelf stable.
As a dietary supplement for adults, take one (1) vegetable capsule daily,
preferably with a meal or as directed by a healthcare practitioner.
$0.79/capsule
[$0.04]
$23.61/30 vegetable capsules
Medium/large vegetable capsule
Free Of: Gluten, Wheat, Dairy, Sugar, Sodium,
Artificial Flavor, Sweetener, Preservatives and Color.
Precaution: Contains soy.
1 vegetable capsule
Lactobacillus plantarum LP229v™ Providing 20 billion
microorganisms.
Other Ingredients: Potato Starch, Vegetable Cellulose, Vegetable Magnesium
Stearate.
APPROVED
Spring Valley™ [Walmart] Acidophilus![]()
Dist. by Wal-Mart Stores, Inc.
1 capsule
1 billion
(At the time of manufactureⓘ)
✔
Found 2.3 billion
Lactobacillus acidophilus
Microbial Contamination: Pass
Disintegration: Pass
Store unopened container at room temperature 59°-86° F (15°-30° C). Refrigerate
after opening.
Adults, take one caplet daily, preferably with a meal.
$0.13/capsule
[$0.13]
$3.96/30 capsules
Large capsule
No Gluten, Wheat, Milk or Milk Derivatives,
Lactose, Sugar, Preservatives, Soy, Artificial Color, Artificial Flavor, Sodium
(less than 5 mg per serving).
1 capsule
Lactobacillus acidophilus [which contains 1 billion active Lactobacillus
Acidophilus Colony Forming Units (CFUs) at the time of manufacture) 5 mg.
Other Ingredients: Cellulose (Plant Origin), Silica, Vegetable Stearic Acid,
Vegetable Magnesium Stearate.
APPROVED
Trunature® [Costco] Advanced Digestive Probiotic
Dist. by Costco Wholesale Corporation
1 vegetarian capsule
10 billion
(At time of manufactureⓘ)
✔
Found 12.1 billion
Lactobacillus acidophilus, Lactobacillus paracasei, Lactobacillus rhamnosus GG, Bifidobacterium
lactis, Bifidobacterium infantis, Lactobacillus salivarius, more...
Microbial Contamination: Pass
Best is stored at 72° F (22° C) or below. Refrigeration not required, but may
extend product shelf life.
Take one (1) capsule once a day.
$0.18/capsule
[$0.02]
$18.49/100 vegetarian capsules
Medium/large vegetarian capsule
1 vegetarian capsule
Xylooligo-
saccharides (XOS) 50 mg
Gluten, soy and dairy free. USP dietary supplement verified seal.
1 vegetarian capsule
Probiotic Blend [Lactobacillus acidophilus, Lactobacillus paracasei,
Lactobacillus rhamnosus GG, Bifidobacterium lactis,
Bifidobacterium infantis, Lactobacillus salivarius, Bifidobacterium bifidum,
Bifidobacterium longum, Lactobacillus reuteri, Lactobacillus casei, more...
APPROVED
Dist. by USANA Health Sciences, Inc.
1 packet
12 billion
✔
Lactobacillus rhamnosus LGG®, Bifidobacterium BB-12®
Microbial Contamination: Pass
Store at room temperature (25° C).
Adults, add the contents of one stick pack to your favorite cold food or
beverage and consume immediately. Take one stick pack every 1-2 days, or as
needed to promote digestive health.
$2.00/packet
[$0.17]
$27.95/14 packets
Powder in packet
Dairy free, sugar free, and gluten free.
1 Stick Pack
Proprietary Blend (Lactobacillus rhamnosus LGG®, Bifidobacterium BB-12®
12 billion CFU).
Ingredients: Mannitol, Inulin, Silicon Dioxide.
APPROVED
Top Pick
for pouchitis; and may reduce bloating in diarrhea-predominant IBS
Visbiome™ High Potency Probiotic
Dist. by ExeGi Pharma, LLC
2 capsules
225 billion
(Guaranteed through "best if used by" date)
✔
Lactobacillus paracasei DSM 24733, Lactobacillus plantarum DSM
24730, Lactobacillus acidophilus DSM 24735, Lactobacillus
delbrueckii subsp. bulgaricus DSM 24734, Bifidobacterium
longum DSM 24736, Bifidobacterium infantis DSM 24737, more...
Microbial Contamination: Pass
Store at 39-46° F, 4-8° C.
Consume 2-8 capsules daily or as directed by your physician.
$1.67/2 capsules
[$0.01]
$199.95/four bottle of 60 capsules (240 capsules total)
Large capsule
Kosher. Visbiome is a Medical food and is
intended to be used under the supervision of a physician.
Precaution: Allergen warning - contains milk.
2 capsules
Each serving (2 capsules) contains at least 225 billion lyophilized lactic acid
bacteria.
Ingredients: Lactic acid bacteria, microcrystalline cellulose, stearic acid,
silicon dioxide, magnesium stearate, and vegetable capsule (hydroxypropyl
methylcellulose).
Women's Products:
APPROVED
Garden of Life® Dr. Formulated Probiotics Once
Daily Women's![]()
Dist. by Garden of Life LLC
1 vegetarian capsule
50 billion
(At expiration date under recommended storage conditions)
✔
Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei,
Lactobacillus paracasei, Lactobacillus bulgaricus, Lactobacillus brevis,
Lactobacillus reuteri, more...
Microbial Contamination: Pass
Store in cool, dry place.
Adults take 1 capsule daily.
$0.98/capsule
[$0.02]
$29.39/30 vegetarian capsules
Large vegetarian capsule
1 vegetarian capsule
Organic prebiotic fiber blend 377 mg
Non-GMO Project verified seal. NSF Certified gluten-free seal. Dairy free.
Soy free. Vegetarian.
1 vegetarian capsule
Women's Daily Probiotic Blend [[Lactobacillus acidophilus, Lactobacillus
plantarum, Lactobacillus casei, Lactobacillus paracasei, Lactobacillus bulgaricus,
Lactobacillus brevis, Lactobacillus reuteri, Lactobacillus salivarius,
Lactobacillus fermentum, Lactobacillus gasseri, more...
APPROVED
GNC Probiotic Solutions Women's![]()
Dist. by General Nutrition Corporation
1 vegetarian capsule
30 billion
✔
Lactobacillus acidophilus (CUL 60), Lactobacillus
acidophilus (CUL 21), Bifidobacterium bifidum (CUL
20), Bifidobacterium animalis subsp. lactis (CUL
34), Lactobacillus gasseri (CUL 09)
Microbial Contamination: Pass
No refrigeration required.
As a dietary supplement, take one capsule daily with food.
$1.00/capsule
[$0.03]
$59.99/60 vegetarian capsules
Large vegetarian capsule
1 vegetarian capsule
Cranberry fruit extract 200 mg
Gluten free. Lactose free.
1 vegetarian capsule
Probiotic Complex [LAB4 Probiotics [Lactobacillus acidophilus (CUL
60), Lactobacillus acidophilus (CUL 21), Bifidobacterium
bifidum (CUL 20), Bifidobacterium animalis subsp. lactis (CUL
34)], Women's Health Blend [Lactobacillus gasseri (CUL 09)]] 30
Billion Cultures, more...
APPROVED
Hyperbiotics® Pro-Women®
Dist. by Hyperbiotics
1 tablet
5 billion
(At time of manufacture. Minimum of 1.5 billion CFU at expirationⓘ)
✔
Found 16.3 billion
Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus acidophilus, more...
Microbial Contamination: Pass
No refrigeration necessary: once opened, store in a cool, dry place.
Daily Maintenance: Take 1 tablet with or without food. Intense support: Take
2-3 tablets twice per day.
$0.70/tablet
[$0.14]
$21.13/30 tablets
Large tablet
1 tablet
Magnesium 2 mg, Cran-Gyn® 250 mg, prebiotic fructooligo-
saccharides (FOS) 25 mg
Timed-release. This formula is dairy free. Vegetarian; non-GMO; yeast free;
no dairy, no soy, no gluten, no wheat, no nuts, more...
1 tablet
Total Carbohydrates <1 g, Total Sugars [Includes 0 g Added Sugars] 0 g,
Magnesium 2 mg, Sodium 10 mg, Proprietary Probiotic Blend® [L. plantarum, L.
fermentum, L. acidophilus, L. reuteri, L. rhamnosus, B. bifidum] 5 Billion
CFU, Cran-Gyn® (D-Mannose, Cranberry Extract) 250 mg, more...
APPROVED
Jarrow Formulas® Jarro-Dophilus® Women![]()
1 veggie cap
5 billion minimum
(At time of consumption, under recommended storage conditions and within best
used before date)
✔
Lactobacillus crispatus, LbV 88, Lactobacillus gasseri, LbV
150N, Lactobacillus jensenii, LbV 116, Lactobacillus
rhamnosus, LbV 96
Microbial Contamination: Pass
Enteric coated disintegration: Pass
Does not require refrigeration and best to store in cool, dry place. Avoid
storage at temperature above 77° F (25° C).
For maintenance, take 1 capsule per day, more...
$0.56/capsule
[$0.11]
$33.57/60 veggie caps
Medium/large veggie cap
No wheat, gluten, soybeans, dairy, egg,
fish/shellfish, or peanuts/tree nuts. Suitable for vegetarians/ vegans. Free of
major allergens.
1 veggie cap
Total Probiotic Count [Lactobacillus crispatus, LbV 88, Lactobacillus
gasseri, LbV 150N, Lactobacillus jensenii, LbV 116, Lactobacillus
rhamnosus, LbV 96] 5 Billion Viable Cells (Minimum).
Other Ingredients: Cellulose and tapioca starch, more...
APPROVED
Top Pick
for vaginal infection
RepHresh™ Pro-B™
Dist. by Church & Dwight Co., Inc.
1 capsule
5 billion
(At time of manufactureⓘ)
✔
Found 4.5 billion
Lactobacillus reuteri (RC-14®), Lactobacillus rhamnosus (GR-1®)
Microbial Contamination: Pass
Do not store above room temperature (77° F).
One (1) capsule daily.
$0.70/capsule
[$0.14]
$21.13/30 capsules
Medium/large capsule
Does not contain: dairy, egg, tree nuts,
shellfish, wheat, peanut, fish, gluten, soy.
1 capsule
Proprietary Probiotic Blend [Maltodextrin, Microcrystalline Cellulose, L.
reuteri (RC-14®), L. rhamnosus (GR-1®), Magnesium
stearate] 5 Billion CFU.
Other Ingredients: Hypromellose, Titanium Dioxide.
Children's Products:
APPROVED
Top Pick
for treating constipation in adults, abdominal pain in children,
and colic in infants
Gerber® Soothe® Probiotic Colic Drops
Dist. by Nestle Infant Nutrition
5 drops [0.2 ml]
100 million
✔
Lactobacillus reuteri Protectis® (DSM 17938)
Microbial Contamination: Pass
Store in a cool, dry place. Maximum 77° F / 25° C.
Use 5 drops once daily.
$0.40/5 drops
[$4.00]
$19.99/0.34 fl oz [10 ml] bottle (approx. 50 servings)
Liquid from bottle
Non GMO.
5 drops
Lactobacillus reuteri Protectis® (DSM 17938) 100 million CFU.
Other Ingredients: Sunflower Oil, Medium Chain Triglyceride Oil, Silicon
Dioxide.
APPROVED
Jarrow Formulas® Jarro-Dophilus® Infant![]()
Dist. by Jarrow Formulas®
10 drops [0.5 ml]
1 billion minimum
✔
Bifidobacterium longum subsp. infantis M-63
Microbial Contamination: Pass
Does not require refrigeration and best to store in cool, dry place. Avoid
storage at temperature above 77° F (25° C).
For newborns to infants up to 6 months old, add 10 drops, once daily, to food,
lukewarm milk, or an infant formula.
$0.34/10 drops
[$0.34]
$10.30/0.51 fl oz [15 ml] bottle (approx. 30 servings)
Liquid from bottle
Gluten free. Dairy free. Suitable for
vegans/vegetarians. No wheat, gluten, soybeans, dairy, egg, fish/shellfish, or
peanut/tree nuts.
10 drops
Bifidobacterium longum subsp. infantis M-63 1
Billion Viable Cells (Minimum).
Other Ingredients: Medium chain triglycerides, tapioca starch, calcium
phosphate, vitamin E (d-alpha tocopherol from sunflower oil; added to maintain
freshness) and citric acid.
APPROVED
SmartyPants® Kids Probiotic Complete - Grape
Dist. by Smarty Pants, Inc.
2 gummies
4 billion
(Total at time of manufactureⓘ)
✔
Found 19.5 billion
Bacillus subtilis DE111, Bifidobacterium coagulans LactoSpore
Microbial Contamination: Pass
No storage conditions listed.
For children 3 years and older, take two (2) gummies daily.
$0.52/2 gummies
[$0.13]
$15.48/60 gummies
Medium/large gumdrop-shaped gummy
American Vegetarian Association [AVA] Certified
Vegan seal. Non GMO. Free of GMOs, gelatin, synthetic colors, artificial
flavors and preservatives, wheat, milk, eggs, soy, gluten, peanuts, tree nut
allergens, fish, shellfish, and salicylates.
2 gummies
Calories 15, Total Carbohydrate 3 g, Sugars 2 g, Sodium 10 mg, Bakers' Yeast
Beta-Glucan Extract (M-Gard) 70 mg, SmartyPants Probiotic Blend [Bacillus
subtilis DE111 3 billion CFU, Bifidobacterium coagulans LactoSpore
1 billion CFU [Total at time of manufacture 4 Billion CFU]] 50 mg, more...
APPROVED
Up4® Probiotic Kids Cubes - Soft + Yummy Vanilla
Melts
Dist. by i-Health, Inc.
1 melt
1 billion
✔
Lactobacillus acidophilus DDS®-1, Bifidobacterium lactis UABla-12®
Microbial Contamination: Pass
Refrigerate for best use.
Chew 1 soft melt daily.
$0.41/melt
[$0.41]
$16.50/40 melts
Large bar-shaped melt
Kosher. Halal. Certified sustainable palm oil.
Preservative free. Gluten free. Sugar free.
Precaution: Contains: milk & soy.
1 melt
Calories 15, Total Fat 1 g, Saturated Fat 1 g, Total Carbohydrate 2 g, Total
Sugars [Incl. 0 g Added Sugars] 0 g, Sugar Alcohol 1 g, Vitamin D2
(Ergocalciferol) 20 mcg, A Proprietary Probiotic Blend Total Cultures [Lactobacillus
acidophilus DDS®-1, Bifidobacterium lactis UABla-12®]
18.5 mg [1 Billion CFU], more...
Oral Health Products:
APPROVED
ProBiora™ Plus - Mint
Dist. by ProBiora Health™, LLC
1 tablet
100 million
✔
Streptococcus uberis KJ2®, Streptococcus oralis KJ3®, Streptococcus
rattus JH145®
Microbial Contamination: Pass
Refrigeration not required.
You can take ProBioraPlus at any time, but taking it at bedtime is more
effective.
$0.48/tablet
[$4.83]
$14.50/30 tablets
Medium circular tablet
Non GMO. Soy, wheat, nut and gluten free.
Contains no artificial sweeteners, flavors or colors. Suitable for vegetarians.
Precaution: Contains milk. Trace levels of milk probiotics
fermentation.
1 tablet
Calories 1, Total Fat 0 g, Sodium 0 mg, Total Carbohydrate 0 g, Protein 0 g,
100 Million CFU ProBiora3® (Streptococcus uberis KJ2®, Streptococcus
oralis KJ3®, Streptococcus rattus JH145®).
Inactive Ingredients: Isomalt, Inulin, more...
Probiotic Drink:
APPROVED
GoodBelly® Probiotics Infused - Watermelon Lime
Dist. by Next Foods
1 bottle [375 ml]
20 billion
(At time of manufactureⓘ)
✔
Found 93.8 billion
Lactobacillus Plantarium 299V
Microbial Contamination: Pass
Keep refrigerated.
No directions stated.
$7.90/bottle
[$0.39]
$63.17/8 bottles
Liquid from bottle
1 bottle
Calcium 130 mg, iron 0.1 mg, potassium 50 mg
Kosher. Non-GMO. Dairy-free. Vegan. No sugars added. Quality Assurance
International Certified organic seal. NSF certified gluten-free seal. USDA
organic seal.
1 bottle
Calories 20, Total Fat 0 g, Saturated Fat 0 g, Trans Fat 0 g, Cholesterol 0 mg,
Sodium 15 mg, Total Carb. 5 g, Dietary Fiber 0 g, Total Sugars (Includes 0 g
Added Sugars) 5 g, Protein 0 g, Vitamin D 0 mcg, Calcium 130 mg, Iron 0.1 mg,
Potassium 50 mg. 20 Billion live & active probiotics, more...
Pet Products:
NOT APPROVED
Rx Vitamins® for Pets RxBiotic
Dist. by Rx Vitamins, Inc.
1 scoop [0.1 g]
2 billion
Found only
390 million CFU (19.5% of listed amount)
Lactobacillus acidophilus, Bifidobacterium longum, Bifidobacterium bifidum,
Bifidobacterium lactis
Microbial Contamination: Pass
Please refrigerate after opening.
Maintenance: 1-2 scoops daily; Restorative: 2-4 scoops daily; With Antibiotics:
2-4 scoops daily.
$0.04/scoop
[$0.02 based on amount listed]
[$0.11 based on amount found]
$24.59/2.12 oz [60.1 g] container (approx. 600 servings)
Powder in container
1 scoop
Fructooligo-
saccharides, larch arabinogalactan (amounts not listed)
Vegetarian. National Animal Supplement Council (NASC) Quality Seal.
1 scoop
DDS ProbioPlus Probiotic Blend [Lactobacillus acidophilus, Bifidobacterium
longum, Bifidobacterium bifidum, Bifidobacterium lactis, Fructooligo-
saccharides, Larch Arabinogalactan 100 mg] 2 billion viable cells, more...
APPROVED
VetriScience® Vetri Mega Probiotic™
Dist. by VertiSciences® Corporation
1 capsule
5 billion
✔
Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium bifidum,
Lactobacillus casei, Lactobacillus brevis, Bifidobacterium longum, Enterococcus
thermophilus
Microbial Contamination: Pass
Refrigerate after opening.
For Dogs: Up to 40 lbs: 1 capsule daily; Over 40 lbs: 2 capsules daily. Give
product after a meal. For cats: Give 1/2 capsule daily mixed with food.
$0.18/capsule
[$0.04]
$22.00/120 capsules
Large capsule
1 capsule
FOS (fructooligo-
saccharides) 75 mg
1 capsule
Calorie Content (calculated): 3,050 kcal/kg; 2 kcal ME/capsule. Moisture (max)
6.0%, Total Microorganisms [Lactobacillus acidophilus, Lactobacillus
plantarum, Bifidobacterium bifidum, Lactobacillus casei, Lactobacillus brevis, more...
APPROVED
Zesty Paws® Probiotic Bites™ - Pumpkin Flavor -
Dogs All Ages
Dist. by Zenwise Health® / Zesty Paws®
1 soft chew
3 billion
✔
Heavy metalsⓘ:
Pass
Bacillus coagulans (Ganeden BC30®), Lactobacillus acidophilus,
Lactobacillus plantarum, Lactobacillus brevis, Lactobacillus fermentum,
Lactobacillus lactis
Microbial Contamination: Pass
Store in a cool, dry place.
Up to 25 lbs.: 1 chew; 26 - 75 lbs.: 2 chews; Over 75 lbs.: 3 chews.
$0.29/soft chew
[$0.10]
$25.97/90 soft chews
Large heart-shaped soft chew
1 soft chew
Pumpkin 800 mg, papaya 200 mg
No ingredients derived from: grain, corn, soy. No artificial flavoring. No
artificial preservatives.
1 soft chew
Moisture (Max) 8%, Pumpkin 800 mg, Papaya 200 mg, Total Microorganisms (Bacillus
coagulans (Ganeden BC30®), Lactobacillus acidophilus, L.
plantarum, L. brevis, L. fermentum, L. lactis). 3 Billion CFU.
Ingredients: Pumpkin, Pea Flour, more...
Unless otherwise noted,
information about the products listed above is based on the samples purchased
by ConsumerLab.com (CL) for this Product Review. Manufacturers may change
ingredients and label information at any time, so be sure to check labels
carefully when evaluating the products you use or buy. If a product's
ingredients differ from what is listed above, it may not necessarily be of the
same quality as what was tested.
The information contained
in this report is based on the compilation and review of information from
product labeling and analytic testing. CL applies what it believes to be the
most appropriate testing methods and standards. The information in this report
does not reflect the opinion or recommendation of CL, its officers or
employees. CL cannot assure the accuracy of information.
Copyright
ConsumerLab.com, LLC, 2021 All rights reserved. Not to be reproduced,
excerpted, or cited in any fashion without the express written permission of
ConsumerLab.com LLC
ConsumerTips™:
What to
Consider When Buying:
Properly labeled probiotic supplements will list the types of bacteria and/or
yeast that are present. Names of the organisms should be listed in italics,
with the genus name capitalized and listed first. The genus name may also be
abbreviated with its first letter (e.g., Lactobacillus acidophilus or L.
acidophilus). Some products will include the specific sub-strain
(e.g. Bifidobacterium lactis BB-12, Lactobacillus
rhamnosus GR-1). There are slight differences between sub-strains of
bacteria, and much of the research has been done on these specific sub-strains.
Keep in mind that products using more general strains may not have the same
intended benefit as products with specific, researched strains.
Some research suggests that it may be more effective to take a probiotic
supplement that contains a mixture of bacterial strains rather than a single
bacterial species. Conversely, it is possible that some combinations of strains
may not work well together, affecting the ability of the organisms to remain
viable. Many products on the market contain untested combinations.
Claimed amounts of cells — Don't get misled by the numbers
It is not uncommon for the amount of active ingredient in a supplement to
decrease over time. Typically manufacturers factor in the expected loss and
display the amount you should expect at the "Best by" date, i.e., the
expiration date. For example, it is not unusual for companies to produce probiotics
which, at the time of manufacture, contain double the amount listed as of the
"Best by" date on the assumption that 50% of the cells die before the
product reaches that date -- particularly if the product is not maintained
under ideal conditions. Even greater losses seem to be anticipated by some
manufacturers: The label on Align notes, for example, that it
"Contains 1x109 (one billion) live
bacteria/CFU when manufactured, and provides an effective level of bacteria
(1x107 [10 million] CFU)
until at least the "best by" date." And Innovix Chewable
Probiotic claims to be "Formulated to provide 6 billion live
cultures at time of manufacture and guaranteed at least 1 billion live cultures
at expiry date." Fortunately, our tests found that each product provided
the amount indicated at the time of manufacture.
Due to this potentially large loss, some products display the number of cells
"at the time of manufacture" which may be very different from what
you'll actually get. ConsumerLab.com frowns upon this practice as it is against
FDA regulations, often exaggerates what is in a product, and does not guarantee
what the product provides. It also makes it difficult for a consumer to make a
fair comparison among products or to gauge the dose that works best. It is best
for products to label the amount of viable organisms expected to be present
through an expiration or "Best By" date. Consumers should try to
purchase products that are well within their expiration date to ensure a higher
number of viable cells.
Interestingly, a study of two VSL #3 products that listed
the same bacterial strains and amounts but were produced by different companies
(one in Italy and one in the U.S.) found differences in the amounts of dead
bacteria contained in each. Each product was packaged in sachets labeled as
containing 420 billion live cells, which they were found to contain. However,
the Italian product was also found to contain 560 - 600 billion dead cells,
approximately 130 - 150% more dead bacteria per sachet than in the U.S.
product. The U.S. product was also found to be significantly more effective in
inhibiting tumor cell growth in in-vitro tests (Cinque, PloS One 2016).
The researchers noted that the quantity of "dead" bacteria is a good
predictor of the quality of the product and its stability, and that the greater
number of dead bacteria in the Italian formulation "may significantly
affect the response and health status of the person ingesting it, especially
children." The authors referenced a previously published review on the
safety of probiotic supplementation which noted that, while "the presence
of dead or injured microbes in commercial products is unavoidable," the
ingestion of heat-killed bacteria has been found to increase the occurrence of
such adverse effects such as diarrhea and other gastrointestinal symptoms (Sanders, Gut Microbes 2010).
Be aware that the differences found in the above noted
study (and several others) became the subject of a lawsuit after the
inventor of the "original" VSL #3 formula, Claudio De Simone, cut
ties with the company selling the product (VSL Pharmaceuticals/ Alfasigma,
Italy) in 2016. He claimed that since that time, the Italian company has been
selling a VSL #3 formula that is different and/or inferior to the original (due
to changes in ingredient quality and production methods) while presenting it as
the same formula used in previous clinical trials (some of which are discussed
in the What It Does section
above). In November, 2018, a jury agreed and awarded
De Simone $15 million in damages. VSL/Alfasigma
contested this verdict, but, on June 20, 2019, the decision was upheld by the
United States District Court for the District of Maryland. The Court ruled that VSL/Alfasigma advertised
falsely with the intent to confuse or deceive the public. It
issued a permanent injunction preventing the defendants from linking their
product with the original De Simone formulation, including citing clinical
studies that were performed using the original De Simone formulation (now sold
by De Simone as Visbiome (ExeGi
Pharma)).
To gain probiotic benefits from yogurt or other
dairy foods, check that it contains organisms known to be probiotic and not
just starter cultures such as Lactobacillus bulgaricus and Streptococcus
thermophilus. Look for products which list specific types and amounts of
probiotic organisms (such as those listed in the table of Evidence above). To the extent that starter
cultures themselves may provide some benefit, check to see if a product
"contains live cultures" or "active cultures" rather than
"made with live/active cultures," which may signal that cultures have
been subsequently destroyed with heat-treatment to eliminate disease-causing
organisms.
Withstanding stomach acid
Some products have an enteric coating or use a protective formulation because
certain probiotic organisms are unable to survive exposure to stomach acid. In
general, most Lactobacillus, Bifidobacterium, and Streptococcus species
do not need enteric coating as they can survive passage through the stomach,
although the percentage which survive may be, according to one study, only 10
to 20% of the original amount when taken without food, while several times more
will survive when enterically coated (Del Piano, Gut Microbes 2011; Del Piano, J Clin Gastroenterol 2010). A study
in a digestive tract model suggested that survival of
non-enterically-coated Lactobacillus and Bifidobacterium in
stomach acid can be improved by taking with a meal containing some fat, or
shortly before such a meal, as opposed to after a meal (Tompkins, Benef Microbes 2011).
A potential downside to an enteric coating is that if the product happens to be
contaminated with unwanted bacteria, yeast, or mold, these are also protected
from your natural defenses. This is another reason why ConsumerLab.com tests
all probiotics for microbial contaminants, which can occur if a product is not
properly made.
Some bacteria naturally sporulate ("hibernate"
within a protective coating) when they are exposed to harsh conditions, and
some researchers postulate that sporulated bacteria are more resistant to the
harsh conditions found in the GI tract. Consequently, another delivery method
is to manufacture probiotics in the form of bacterial spores. This is most
often used for bacteria of the genus Bacillus including B.
coagulans (previously called Lactobacillus sporogenes),
although clinical evidence supporting B. coagulans remains
preliminary. A tablet containing 360 million cells of L. sporogenes taken
daily for three months significantly reduced total and LDL cholesterol in a
small study of men with high cholesterol (Mohan, Indian J Med Res 1990), but this study
was neither blinded nor placebo controlled. One clinical study did not find
improvement in diarrhea in children when given a daily dose of 240 million
cells of L. sporogenes (Dutta, Trop Med Int Health 2011). A branded
version of B. coagulansis is sold as LactoSpore by Sabinsa Corp.
The LactoSpore.com website notes studies suggesting that a daily dose of 75 to
600 million cells of this strain may improve diarrhea and 300 to 750 million
cells may improve constipation in adults, although this does not appear to have
been published in a peer-reviewed journal.
L. bulgaricus and S. thermophilus, as well as Leuconostoc and Lactococcus species
are commonly found in yogurt because they are used as starters for dairy
products. It was once believed that these microorganisms could not survive
passage through the stomach, though new research suggests otherwise (Mater, FEMS Microbiol Lett 2005; Elli, Appl Environ Microbiol 2006). In
addition to surviving gastrointestinal transit, these strains produce large
quantities of lactic acid which may limit the growth of unfriendly bacteria and
help in the prevention and treatment of diarrhea. In addition, by converting
the lactose in dairy products into lactic acid (which may occur more in the gut
than in the product), S. thermophilus and L.
bulgaricus help make dairy food more tolerable for people with lactose
intolerance. As noted earlier, yogurts and other dairy products specifically
marketed as probiotics are often fortified with additional species to provide
additional benefit.
Different names for the same strain
Occasionally, bacteria strains may be re-classified, or companies may create a
trademarked name for a particular strain for marketing purposes. For example,
there has been some confusion over the name of the probiotic bacteria found
in Activia yogurt (Dannon), once listed as Bifidobacterium
animalis DN-173 010. Currently, it is listed on Activia labels
in the U.S. as Bifidobacterium lactis. This is because Bifidobacterium
animalis and Bifidobacterium lactis were once
considered to be two separate species of bacteria, but Bifidobacterium
lactis is now re-classified as a subspecies of Bifidobacterium
animalis (Masco, Int J Syst Evol Micro 2004).
So while the same bacteria is being used, it is now officially known as Bifidobacterium
animalis, subsp. Lactis, strain DN-173 010 — sometimes shortened
to Bifidobacterium lactis. Dannon has trademarked the name Bifidus
Regularis® for this strain, so you may also see it listed this way on the
label.
Interestingly, Bifidobacterium animalis, subsp. Lactis,
strain DN-173 010 has a different name when listed on Activia labels
in other parts of the world. In Canada, for instance, the same strain is
trademarked as BL Regularis™ and was assigned a new name, "Bifidobacterium
lactis CNCM I-2494" in the European registry for microorganisms, while
in England, the strain in called Bifidus ActiRegularis™.
See the What They Do section
for more on the evidence for this strain.
Color of probiotics
Most probiotic material in powders, capsules, and tablets, is white or
off-white. A pink color can be seen in some lactobacillus-containing
probiotics, likely due to a chemical reaction with a preservative, such as
sodium ascorbate (Kurtmann, Cryobio 2008).
This is not harmful.
Kombucha
·
How sugars are labeled: New FDA labeling rules for commercial
kombucha beverages (to be implemented by January 1, 2020 or January 1, 2021,
depending on company size) require that the amount of sugar present after
fermentation be listed as "Sugars" while all sugar used for
fermentation or added later for flavoring be listed on labels as "Added
Sugars." "Total Sugars" will include both of these amounts. More
information about what new kombucha labels will be required to list, and an
example, label can be found here.
·
Alcohol in kombucha: Kombucha contains a small amount of
alcohol (typically less than 0.5% -- as compared to beer which contains about
5%). The FDA does not require beverages containing less than 0.5% alcohol by
volume to be labeled as containing alcohol. Be aware that some commercial
kombuchas have been found to contain up to 2.5% alcohol by volume (Ebersole, J AOAC Int 2017; Chicago Tribune 2015),
although a later study of 18 commercial kombucha beverages (including brands
such as GT's and Kevita -- although not the flavors tested in this Review)
found the alcohol by volume in products to range from 1.12—2.00%. The alcohol
content in commercial kombucha products can increase slightly over time (up to about
21 days), although refrigerating unopened bottles may slow or negate this
increase (Mohsen, Food Analytic Methods 2017).
Due to consumer concerns over the alcohol and sugar content of kombucha
beverages, several lawsuits against retailers of kombucha beverages have
been filed and/or settled.
For more about the regulation of alcohol in kombucha see the U.S. Alcohol and
Tobacco Tax and Trade Bureau's Kombucha Information and Resources
website.
·
Storage: Kombucha should be refrigerated at all times. If exposed
to warm or hot temperatures, the fermentation continues rapidly and carbon
dioxide can build up quickly, resulting in excess carbonation upon opening or
an exploding or broken bottle (Kombucha Brewers International 2016).
What to Consider When Using:
Probiotics are measured in terms of the number of viable (i.e., living or hibernating)
organisms per dose and stated as "units" or "colony forming
units" (CFU). The recommended intake for probiotic supplements varies by
strain and use, but tends to be about 1 billion to 10 billion cells (or CFUs)
per day. These amounts may be written on the label as 1 x 109 or 109 for one billion units, and 1 x 1010 or "1010 for ten billion units. Although less
common, some strains are effective at as little as 50 million viable cells per
day, while you may need more than 1 trillion cells of other strains to receive
clinical benefit (O'Mahony, Gastroenterology 2005).
In general, probiotics will only survive a few days to
about 3 weeks in your system after you stop taking them (Raish, Appl Environ Microbiol 2006; Kullen, J Nutr 1997), and so should be taken
on an ongoing basis for general benefits, or for the period of time indicated
by clinical studies for a specific use (see dosage information below).
Dosage:
The types and number of organisms taken as probiotics depend on the use for
which they are intended:
·
For irritable bowel syndrome (IBS) and functional abdominal pain
In adult women, one hundred million to 1 billion cells daily of Bifidobacterium
infantis 35624 has been shown to reduce abdominal pain, bloating, and
bowel movement difficulty, but it does not reduce the frequency of bowel
movements (Whorwell 2006). In adult men and women, a
4-strain combination product (sold as Symprove in
England) of Lactobacillus rhamnosus NCIMB30174, Lactobacillus
plantarum NCIMB 30173, Lactobacillus acidophilus NCIMB 30175,
and Enterococcus faecium NCIMB 30176 in a water-based
suspension of barley extract with 10 billion live organisms per 50 mL daily
reduced pain and improved bowel habits but not bloating or overall quality of
life (Sisson Aliment Pharmacol Ther 2014).
An 8-strain combination product of 450 billion live organisms from Bifidobacterium, Lactobacillus,
and Streptococcus genera VSL#3, now sold as Visbiome) has also
helped with IBS (Kim 2003) but be aware that the formula currently sold as VSL#3 may be different than
the formula used in this study. Four billion cells daily of Saccharomyces
cerevisiae CNCM I-3856 reduced abdominal pain and discomfort after 4
weeks (de Chambrun, Digestiv and Liver Dis
2014). A tablet containing 2 billion spores of B. coagulans MTCC
5856 taken once daily at least 30 minutes before a meal (typically breakfast)
decreased many symptoms in people with diarrhea-predominant IBS also receiving
standard medical treatment (Majeed, Nutr J 2016).
In children, 3 billion cells of Lactobacillus GG twice per day
reduced frequency and severity of pain (Ruggiero 2010). Intensity of pain in children
with functional abdominal pain was reduced with 200 million cells daily
of Lactobacillus reuteri DSM 17938 (Romano, J Paediatr Child Health 2010).
Frequency of colic in some infants was also reduced with drops containing 100
million cells daily of the same strain (sold as Gerber Soothe Colic Drops)
(Indrio, JAMA Pediatr 2014; Sung, BMJ 2014; Savino, J Pediatr 2017).
·
For diverticular disease
Adding a probiotic to standard drug therapy has reduced the recurrence of
symptoms and, in acute situations, reduced pain. Lactobacillus casei subsp.
DG, 24 billion organisms daily (Tursi, Aliment Pharm Ther 2013) or Lactobacillus
reuteri ATCC PTA 4659, 500 million organisms twice daily (Petruzziello, Int J Colorect Dis 2019).
·
For the treatment of antibiotic-related diarrhea
Although it has been recommended to start taking probiotic supplements as soon
as symptoms appear, more recent studies show that probiotics can be taken
starting the first day of oral antibiotic treatment and continued for 1 to 2
weeks after the completion of antibiotic therapy -- although one study suggests
that this continuation can delay restoration of normal bacteria in the gut (Suez, Cell 2018). It may be advisable to take
probiotics and antibiotics at least 2 hours apart to reduce the possibility of
the antibiotic killing the probiotic organisms. Ten to 20 billion cells
of Lactobacillus GG (Culturelle) daily (Osterlund 2007), and 50 to 100 billion cells
of a combination of Lactobacillus acidophilus CL1285 and Lactobacillus
casei LBC80R (Gao, 2010) have been shown to be reduce the
incidence of antibiotic-related diarrhea, as have 17 billion cells daily of a
combination of L. acidophilus NCFM, L. paracasei Lpc-37, B.
lactis Bi-07 and B. lactis Bl-04 in equal parts (HOWARU Restore) (Ouwehand, Vaccine 2013). Lacidofil®
Strong (Lallimand; sold in U.S. by Xymogen) taken twice a day has
shortened the duration of diarrhea in adults, with each dose consisting of 3.8
billion cells of Lactobacillus rhamnosus R0011 and 200 million
cells of Lactobacillus helveticus R0052, (Evans, Br J Nutr 2016). A combination of Lactobacillus
GG (5.2 billion cells daily) with Bifidobacterium lactis (Bb-12)
(5.9 billion cells), and Lactobacillus acidophilus (La-5) (8.3
billion cells), in a yogurt has been effective in children (Fox, BMJ Open 2015). Products containing multiple
species of organisms may be somewhat more effective than those with a
single species (Johnston, Ann Int Med 2012).
If diarrhea symptoms persist for several days, see a physician. Also, 500 mg
twice daily of S. boulardii during a week of antibiotic
treatment and for a week afterward (Kabbani, Gut Microbes 2017)
·
For rotaviral diarrhea in infants and children up to age 3
Doses of up to 10 billion cells of Lactobacillus GG and Lactobacillus
reuteri may significantly reduce the diarrheal phase of infection.
·
For chemotherapy-induced diarrhea
Ten to twenty billion cells of Lactobacillus GG (Culturelle)
daily has shown to be effective (Osterlund 2007).
·
For traveler's diarrhea
Two billion viable cells of Lactobacillus GG taken by mouth
daily starting 2 days before travel and continuing throughout the trip may
reduce the likelihood of traveler's diarrhea (Hilton 1997). Saccharomyces boulardii 250
mg (as in FloraStor) to 1 gram by mouth daily begun 5 days before
travel and continued for the duration may reduce traveler's diarrhea, with the
larger dose possibly more effective (Kollaritsch 1993).
·
For proton pump inhibitor (PPI) induced bloating
12 billion cells of L. paracasei F19 taken twice daily three
times a week has been shown to prevent bloating and flatulence occurring with
continued PPI use (Compare, Digestive and Live Dis,
2015).
·
For reducing cold and flu symptoms
- Adults: One billion viable cells of a combination of Lactobacillus
plantarum HEAL 9 (DSM 15312) and Lactobacillus paracasei 8700:2
(DSM 13434) by mouth daily for 12 weeks during cold season was shown to reduce
the incidence and duration of colds (Berggren 2011), although not all studies using
this combination have found a benefit (Ahren, J Nutr 2020).
- Children: A combination of Lactobacillus acidophilus NCFM
(2.5 billion cells and Bifidobacterium animalis subsp lactis Bi-007
(2.5 billion cells) taken as a powder mixed with milk twice a day during colder
months (Leyer 2009). Also, taken as soon as a
household member is sick, to reduce severity acute respiratory infections
caught by children, a daily dose of Lactobacillus acidophilus DDS-1
(1 billion cells) and Bifidobacterium lactis UABLA-12 (4
billion cells) with a fructooligosaccharide prebioitc (50 mg) (Sold as UP4
Junior, UAS Laboratories)(Gerasimov, Eur J Clin Nutr 2015).
·
For throat infection
In children, a lozenge containing 1 billion cells of S. salivarious K12
taken daily for three months has been shown to significantly reduce the
occurrence of streptococcal and viral throat infections (Pierro Drug Healthc Patient Saf 2014).
·
For treating periodontitis
A lozenge containing 100 million cells of each of two strains of Lactobacillus
reuteri, DSM17938 and ATCC PTA5289 (Prodentis from
BioGaia, Sweden, sold in the U.S.and Canada as GUM PerioBalance) taken twice daily after a dental scaling and
disinfection may be helpful in patients with moderate to severe periodontitis (Teughels, J Clin Periodont 2013).
·
For vaginal bacterial infections
One capsule daily containing over one billion viable cells of both L.
rhamnosus GR-1 and L. fermentum RC-14, respectively,
(as in Jarrow Formulas femdophilus) has been taken orally and shown
to reduce colonization of the vagina by potential pathogenic bacteria and yeast
(Reid 2003). Vaginal suppositories (which are
not considered supplements in the U.S.) containing approximately 1
billion Lactobacillus organisms have also shown therapeutic
benefit.
·
For mastitis
90 billion cells of either Lactobacillus fermentum CECT5716
or Lactobacillus salivarious CECT57132 taken daily for 21 days
has been shown to reduce breast pain and resolve infection in nursing mothers
with mastitis, as well as reduce the rate of recurrence (Arroyo Clin Infect Dis 2010).
·
For cholesterol-lowering
LDL-lowering: Lactobacillus reuteri NCIMB 30242 (2 billion
cells with breakfast and dinner) (Jones, Eur J Clin Nutr 2012), or E. faecium M-74
(2 billion cells daily), or Lactobacillus acidophilus La5
and Bifidobacterium lactis Bb12 (1.2 billion cells each) (Nabavi, J DairySci 2014); LDL and triglyceride-lowering: Lactobacillus
curvatus HY7601 and Lactobacillus plantarum KY1032 (5
billion cells each) (Ahn, Atherosclerosis 2015)
or L. fermentum ME-3. (8 billion cells) (Mikelsaar, BMC Nutrition 2015).
·
For reducing anxiety
3 billion cells of B. longum R0175 with Lactobacillus
helveticus R0052 taken daily with breakfast has been shown effective
in a 30-day study (Messaoudi, Br J Nutr 2011).
·
For reducing risk of depression
A total of 5 billion cells from two forms of Bifidobacterium (bifidum W23
and lactis W52) and five forms of Lactobacillus (acidophilus W37, brevis W63, casei 56, salivarius W24,
and lactis W19 and W58) taken as a powder mixed with water or
lukewarm milk before bed reduced negative thoughts associated with sadness (Steenbergen, Brain Behav Immun 2015).
·
For weight and fat loss
Doses of 1.4, 16, or 100 billion cells daily of Lactobacillus gasseri SBT2055
(LG2055) in a fermented milk product have been used in preliminary research.
These doses appear to have similar effects, except that the lower doses of 1.4
or 16 billion cells do not appear to provide the same subcutaneous fat loss as
the higher dose (Kadooka, Eur J Clin Nutr 2010; Kadooka, Br J Nutr 2013). In obese women, a
probiotic providing 162 million cells daily of Lactobacillus rhamnosus CGMCC1.3724
(also called LPR) along with 300 mg of a prebiotic mix of oligofructose and
inulin divided into two capsules has been successfully used (Sanchez, Br J Nutr 2013). A probiotic yogurt
given for 8 weeks (300 grams per day providing about 1.2 billion cells each
of Lactobacillus acidophilus La5 and Bifidobacterium
lactisBb12) appeared to reduce weight by nearly 4 lbs as well as slightly
reduce LDL and total cholesterol (Nabavi, J DairySci 2014). Fifty billion cells
of Bifidobacterium brevis B-3 given as a capsule daily
appeared to cause a small reduction in fat in overweight adults in Japan (Minami, J Nutr Sci, 2015).
·
For treating allergy
2 billion cells of Lactobacillus paracasei LP-33 daily may
further reduce the impact of allergy symptoms on the quality of life in people
with grass pollen allergy already taking an antihistamine (loratadine 10 mg) (Costa, Eur J Clin Nutr 2014).
·
For reducing risk of eczema and, possibly, neuropsychiatric
disorders (ADHD and Asperger Syndrome) in children
10 billion cells daily of Lactobacillus GGCulturelle) to pregnant
mother for 4 weeks before expected delivery and to infant for 6 months after
delivery or mother if breast-feeding (Partty, Ped Res 2015), or, to reduce the risk
of eczema, 6 billion cells daily of Lactobacillus rhamnosus HN001
(sold as HOWARU® Rhamnosus) taken by mothers from week 35 of
pregnancy to 6 months after delivery and by infants from birth to age 2 years (Wickens, Pediatr Allergy Immunol 2018).
·
For small pets (dogs and cats)
Probiotics may help stimulate immune function. Typical amounts shown in studies
for immune stimulation in cats and puppies tend to range in the hundreds of
millions. To colonize the gut with a probiotic, or when treating bigger dogs,
larger doses may be appropriate.
Storage
It is advisable to keep probiotics out of heat and light. After being opened,
they should also be kept away from moisture to prevent organisms from becoming
activated and then dying. Although not always a requirement, labels on some
probiotic products suggest that they be refrigerated to prolong their shelf
life. These requirements depend on the organism, the formulation and pill type,
and the packaging, such as bottled capsules as opposed to blister packs.
Probiotic yeast and some of the spore-forming bacteria, such as Bacillus
coagulans, generally do not require refrigeration. Check the specific
storage recommendations on each product, (shown in the Ingredients listings) as they may vary.
Kombucha
Be aware that kombucha contains a small amount of alcohol, typically, less than
0.5% by volume, but amounts up to 2.5% have been found (Mohsen, Food Analytic Methods 2017; Chicago Tribune 2015).
Animal studies suggest kombucha may have a blood sugar lowering effects; use
with caution if you have diabetes or take blood-sugar lowering medication (Aloulou, BMC Complement Altern Med 2012).
Pathogenic bacteria can form in kombucha which is not properly made or stored.
Allowing kombucha to ferment for too long (more than 7 — 10 days) can increase
the risk of pathogenic bacterial contamination and increase the acetic acid
content of kombucha. Several cases of muscle breakdown, lactic acidosis and
other forms of metabolic acidosis, sometimes fatal, have been associated with
the consumption of kombucha, typically in people consuming home-made kombucha (Holbourn, BMJ Case Reports 2017; Kole, J Intens Care Med 2009; Derk, Clin Rheumatol 2004; CDC 1995).
Due to the presence of alcohol and the risk of pathogenic bacteria, women who
are pregnant or breastfeeding and people with weakened immune systems, should
avoid kombucha or consult a physician before using.
People with histamine intolerance or with low activity of an enzyme called DAO
may have an odd reaction to kombucha which can mimic drunkenness (Kombucha Brewers International 2016). (Also
see the this CL Answer for about
more about DAO, histamine intolerance and fermented foods).
To further assist consumers, ConsumerLab.com is licensing its flask-shaped CL
Seal of Approved Quality (see The CL
Seal) to manufacturers for use on labels of products that have
passed its testing. ConsumerLab.com will periodically re-evaluate these
products to ensure their compliance with ConsumerLab.com's standards.
All About Prebiotics:
As mentioned in the What They Are section of this
review, prebiotics are plant-based complex sugars that promote
the growth of probiotic bacteria in the gastrointestinal tract. They
are not fully digested by the human body but are used as "food" by
the bacteria in the colon. The most common prebiotic ingredients you will see
listed on supplements are inulin and fructo-oligosaccharides (FOS or
oligofructose). Inulin is not one molecule but a blend of molecules, many of
which can be hydrolyzed to yield FOS. Both FOS and inulin have similar effects
in the body.
Although inulin and FOS are sometimes promoted for improving cholesterol
levels, the evidence is mixed. Several short term studies using high doses
(10 — 20 grams) found a modest reduction in total and LDL-cholesterol; however,
other studies, including a longer-term, 6-month study, have not found a
cholesterol-lowering effect (Williams, Br J Nutr 2002; Forcheron, Metabolism 2007).
A small study among prediabetic men and women who
participated in calorie-restricted diet found those who took 30 grams of inulin
(10 grams taken with food or water 3 times per day) for approximately 4
months lost significantly more weight than those who took a
placebo, with an average loss of about 17 lbs versus 11 lbs, respectively.
Those who took inulin also experienced significantly greater reductions
liver fat (higher levels of which are associated with insulin
resistance); however, there were no significant improvements in blood
sugar levels or insulin resistance (Guess, Nutri and Metabol 2015). The inulin
(Orafti® Synergy1,Beneo-Orafti Inc.) can be found in products such as Jarrow
Formulas Inulin FOS. To avoid gastrointestinal discomfort,
participants began with a dose of 10 grams per day and increased the daily dose
by 10 grams every two weeks until reaching 30 grams per day.
The same prebiotic was given at a dose of 8 grams daily (mixed
in water and consumed before dinner) in a small study among overweight and
obese children. Sixteen weeks of treatment resulted in modest reductions in
trunk body fat, waist circumference, body mass index, and fasting insulin --
however the effects were not statistically significant compared to placebo (Nicolucci, FASEB J 2015).
During the final week of the study, 11- and 12-year olds treated with the
prebiotic chose a lower calorie buffet breakfast than those who had received
the placebo — although this did not occur among younger children (Hume, Am J Clin Nutr 2017).
To minimize possible flatulence and bloating, only half the dose was taken
during the first two weeks of the study.
A small study among overweight and obese men and women
found that taking 10 grams of inulin (Orafti, Beneo-Orafti) twice daily
mixed into foods or beverages improved insulin resistance compared to
consumption of an equal amount of cellulose which, unlike inulin, is not highly
fermentable. A similar effect was achieved with 10 grams twice daily of an
inulin-propionate ester (IPE, 7.3 grams of inulin with 2.7 grams of propionate
ester), although the inulin-only product achieved greater effects on the gut
bacterial population while IPE had a greater effect on reducing proinflammatory
interleukin-8 levels (Chambers, Gut 2019).
(Propionate and butyrate are short-chain fatty acids, or SCFAs, produced by the
fermentation of inulin and other fiber in the gut and may play important roles
in the health effects of prebiotics.)
There is some evidence that a daily dose of 10 grams of inulin can improve
measures of glycemic control in women with type 2
diabetes (Gargari, Diabetes Metab J 2013).
A small study in men and women with constipation (defined
as having 2 to 3 bowel movements per week for six months or more) found that 4
grams of chicory inulin (Orafti, Beneo) added to a hot or room temperature
beverage and consumed three times per day (providing a total of 12 grams of
inulin daily) modestly increased the frequency of bowel movements compared to a
placebo drink (4 vs. 3 per week, respectively). Self-reported symptoms of
bloating and abdominal discomfort were similar for both groups; however, those
consuming inulin reported having more gas (Micka, Int J Food Sci Nutr 2016).
The study was funded by the makers of Orafti.
Prebiotics are sold in powder, tablet and capsule form, typically providing 2
to 4 grams of inulin or FOS per daily serving — the amount shown to support the
growth of healthy bacteria in the gastrointestinal tract (Bouhnik, Nutr Res 2007; Dehghan, Health Promot Perspect 2013).
However, because this is more ingredient than can fit in a single pill, it is
necessary to take several pills to get this amount of inulin or FOS. Prebiotics
are sometimes added as ingredients in probiotic supplements, but
usually in low doses (less than a quarter of a gram per pill), or as part of a
"blend" in which the exact amount of prebiotic is not even listed.
Be aware that doses of 10 grams or more of inulin or FOS can produce
gastrointestinal discomfort, gas and bloating. To help avoid, try starting with
a smaller dose and increasing it over a period of a few weeks (Guess, Nutri and Metabol 2015).
(See the Results section of this review for tests
of probiotic products that also contain prebiotics — this is usually listed in
the last column, Other Notable Ingredients).
The prebiotic ingredient found in supplements is usually derived from chicory
root, which is extremely rich in inulin. A variety of foods, in addition to
chicory, can provide good amounts of FOS, or more broadly, inulin. For example,
as shown in the chart below, 100 grams of banana (a small banana) provides 500
mg of inulin (all of which is FOS). Similar sized servings (100 grams) of some
other foods, like wheat flour or wheat bran, can provide several times that
amount. Inulin and FOS are sometimes also added to processed foods to improve
taste (they are slightly sweet) and texture. In all, the average American diet
provides about 2.6 g of inulin (2.5 g of FOS) daily -- most of which comes from
wheat (70%) and onions (25%), so the foods you eat may already provide all the
prebiotics you need (Moshfegh, J Nutr 99).
Food sources of prebiotics: amounts of inulin* and oligofructose
(in grams) per 100 grams serving.
|
Food Source |
Inulin* g/100 g |
Oligofructose (FOS) g/100 g |
|
Banana (raw) |
0.5 |
0.5 |
|
Asparagus (boiled) |
1.7 |
1.7 |
|
Chicory root |
41.6 |
22.9 |
|
Dandelion greens |
13.5 |
10.8 |
|
Garlic (raw) |
12.5 |
5.0 |
|
Garlic (dried) |
28.2 |
11.3 |
|
Jerusalem artichoke |
18.0 |
13.5 |
|
Leeks (raw) |
6.5 |
5.2 |
|
Onions (raw) |
4.3 |
4.3 |
|
Onions (cooked) |
3.0 |
3.0 |
|
Wheat (bran — raw) |
2.5 |
2.5 |
|
Wheat (flour — baked) |
2.4 |
2.4 |
*Inulin amounts include
oligofructose (FOS) and other prebiotic compounds
Source: Presence of Inulin and
Oligofructose in the Diets of Americans, Ju Nutr 1999
Another type of prebiotic is galactooligosaccharide (GOS), which is
commercially produced through the enzymatic conversion of lactose, from milk.
Several forms of GOS are sold, primarily in Asia and Europe. One form, marketed as Bimuno®-GOS, is produced
with an enzyme from bifidobacteria and, perhaps not surprisingly, seems to be
particularly effective at increasing the gut population of Bifidobacterium,
which is thought to be beneficial to the immune system. Levels of
Bifidobacterium in the colon tend to decline with age. A clinical study in
adults age 65 to 80 found those who consumed 5.5 grams of Bimuno®-GOS powder
mixed with a glass of water daily for 10 weeks had significant increases
in Bifidobacterium compared to when they were given placebo
for 10 weeks (Vulevic, B J Nutr 2015).
The prebiotic also lead to significant increases in several anti-inflammatory
immune system markers (interleukin-8 and -10) and natural killer cell activity,
as well as decreases in certain markers of inflammation. The study was funded
by the maker of this product (Classado BioSciences).
Another prebiotic is punicalagin, a polyphenol isolated
from Indian pomegranate. This non-fermenting prebiotic is an ingredient
in Daily Synbiotic by Seed in which punicalagin forms an outer
capsule surrounding an inner capsule that contains up to 24 probiotic strains.
According to the manufacturer, the punicalagin outer capsule helps provide a
barrier to oxygen, moisture, and heat (which can damage the probiotic strains)
and can be broken down by gut microbes once ingested. Some of the probiotic
strains included in the product have shown health benefit in clinical research.
For instance, Bifidobacterium longum BB536, Lactobacillus
plantarum LP01 and Bifidobacterium breve BR03 have
shown benefit for hard stools and gastrointestinal conditions such as irritable
bowel syndrome (Del Piano, J Clin Gastroenterol
2010; Ogata, Biosci Microflora 1997; Saggioro, J Clin Gastroenterol 2004). Lactobacillus
salivarius LS01, Bifidobacterium lactis CECT8145
and Lactobacillus casei CECT9104, in combination with some of
the previously mentioned strains, have been shown to be helpful for atopic
dermatitis (Navarro-Lopez, JAMA Dermatol 2018; Iemoli, J Clin Gastroenterol 2012). However,
since there does not appear to be research on the effects of the exact
combination of punicalagin and probiotic strains found in Daily
Synbiotic, it is unclear if the synbiotic combination has significant
health benefit. Also, the amount of prebiotic in the suggested daily serving is
only 400 mg, which may be too little to be beneficial. Prebiotics are typically
taken in daily doses of 2 to 6 grams.
Concerns and Cautions:
There
are no known safety issues with most probiotic bacteria at appropriate doses in
healthy people but some people occasionally notice a temporary increase in
digestive gas and, as described below, there can be allergic reactions.
Rarely, serious infections have occurred in
people with severe illness or weakened immune systems who consumed or were exposed
to probiotic organisms.
Allergic reactions
Individuals with milk allergies should be aware that probiotics which contain
lactose fermenting bacteria (e.g., Lactobacilli and Bifidobacterium)
are typically grown on media containing milk-derived nutrients. Such products
may potentially contain residual milk proteins, even if they claim to be free
of milk or dairy. Some, but not all, products note this on their labels. It may
be prudent to assume that any lactose fermenting probiotic can potentially contain
residual milk proteins and be allergenic.
Saccharomyces cerevisiae (Brewer's yeast) and Saccharomyces
boulardii should not be taken by people with an allergy to these
yeasts. At least one case of allergic rash from Saccharomyces boulardii has
been reported (Kartal, Clin Trans Allergy 2014; Airola, Ann Allergy Asthma Immunol 2006). A rare
gastrointestinal allergic reaction has been reported in an infant with a prior
diagnosis of food protein-induced enterocolitis syndrome who was given S.
boulardii (Kelesidis, Therap Adv Gastroenterol 2012).
[Note: Saccharomyces cerevisiae should not be taken with the
drug Demerol (meperidine) or with drugs which are MAO inhibitors as it may
cause dangerously high blood pressure (Zisook, Psychosomatics 1985; Brown, Ann Pharmacother 1988).]
Undesirable yeasts and molds may also occur in contaminated probiotics,
representing other potential allergens. ConsumerLab.com checks all
probiotics which it reviews for these and other microbial contaminants and will
not Approve those which test positive.
In 2013, an individual reported to ConsumerLab.com developing hives and itching
for over a month after taking MD PureBiotic, which are vegetarian
capsules sold in a bottle.
Rarely, allergic reactions, including anaphylaxis, have been reported due to
the prebiotic inulin, including anaphylaxis, have been reported;
people with a history of allergy to artichoke may be more likely to have an
allergic reaction to inulin (Gay-Crosier, N Eng J Med 2000; Franck Int Arch Allergy Immunol 2005).
Some research has suggested that that fermented dairy
products — particularly cheeses -- may trigger migraine headaches due to the
compounds tyramine and histamine which occur in such products due to the
fermentation process. However, the connection between these compounds and
migraine has not been well established (Pascual, Cephalgia 2010).
Kefir and yogurt have been reported to contain much smaller concentrations of
these compounds relative to certain cheeses (Linares, Front Microbiol 2012) -- although
amounts could add up due to typically larger serving sizes of kefir and yogurt
than cheese. Some people have reported a decrease in migraines when eliminating
dairy or fermented foods from their diet (Finocchi, Neurol Sci 2012). The National
Headache Foundation recommends that people with migraine avoid fermented foods,
and limit consumption of yogurt to ½ cup per day (National Headache Foundation 2007).
Interestingly, a 14-strain probiotic (Bio-Kult) was found to reduce migraine frequency and severity in
people with episodic or chronic migraine.
Bacterial/fungal infections
In a relatively small number of cases (mainly among individuals who were
severely ill, immunocompromised, enterically fed and/or with central venous
lines) probiotic use has caused bacteremia or fungemia (presence of bacteria or
fungi in the blood) or pathological infections resulting in severe illness.
Examples include the following:
A 36-year-old woman in Australia with alcoholic cirrhosis of
the liver, colitis, and a pre-existing heart abnormality died from endocarditis
(inflammation in the lining of the heart) caused by re-occurring Lactobacillus
rhamnosus bacteremia; she had taken a commercially available probiotic
containing Lactobacillus acidophilus, Lactobacillus
rhamnosus and Saccharomyces cerevisiae for two months
before she became ill from the infection (Naqvi, IDCases 2018).
Supplementation with Bacillus subtilis has been reported to
cause bacteremia and sepsis (a severe bacterial infection in the blood) in
people with compromised immune systems due to cancer (Richard, Eur J Clin Microbiol Infect Dis 1988; Oggioni, J Clin Microbiol 1998).
Bacillus subtilis and Enterococcus facieum have
caused serious infections in people with compromised immune systems who were
exposed to these bacteria through physical contact, such as through a wound or
a urinary catheter (Thomas, J Neurol Neurosurg Psychiatry 1991; Selleck, Microbiol Spectr, 2019).
Cases of fungemia have also been associated with the use of use of probiotics
containing the yeast Saccharomyces cerevisiae (S.
cerevisiae) and S. boulardii (which was originally
misclassified and is actually strain of S. cerevisiae): A 74-year-old man who had taken a probiotic supplement
containing Saccharomyces boulardii for seven years
developed S. cerevisiae fungemia (confirmed by blood culture)
during hospitalization and treatment for an unrelated condition (bile duct
infection) (Fadhel, Medical Mycology Case
Reports 2018). An 82-year old woman in
Argentina with diabetes and Alzheimer's disease who had been taking an oral
probiotic containing S. boulardii for six months to help
treat C. difficile-associated diarrhea developed fungemia after a
hospital procedure (stomach feeding tube placement); laboratory tests showed
the yeast found in her blood appeared similar to that in the probiotic
supplement (Landaburu, Rev Argent Microbiol
2019). Capsules or packets containing these probiotics
should not be opened in the vicinity of patients with central lines, and
healthcare workers should change gloves after handling these products.
In 2014, a premature infant given a probiotic powder died from a fungal
infection (mucormycosis) resulting from a contaminating mold (Rhizopus
oryzae) in the probiotic, prompting the U.S. FDA to issue a general warning about the risk of
fungal infection from probiotics given to people with weakened
immune systems. However, HIV-positive adults have taken probiotics for up to 3
weeks without side effects.
Eosinophilic syndrome
In 2012, two cases of severe eosinophilic syndrome (an autoimmune disease)
occurring in the same month and city were associated with the use of a
probiotic supplement between two and four weeks prior to symptom onset (Mendoza, Case Reports Rheum 2012). The
patients did not remember the name of the product, but both recalled use of a
"new brand of probiotics described as an 'extra strength concentration' in
a boxed blister pack, purchased over-the-counter in a Philadelphia metropolitan
area pharmacy." In both cases, symptoms were sudden in onset and included
malaise, weakness in the arms or legs, and numbness. Both patients required
treatment with IV corticosteroids followed by immunosuppressive therapy for
several months. Although general symptoms abated, both patients suffered
permanent motor and sensory deficits in the lower extremities.
If you have a reaction to a probiotic, let us know — and please provide
details.
References:
Benyacoub J.,et al (2003) Supplementation of
Food with Enterococcus faecium (SF68) Stimulates Immune
Functions in Young Dogs. J. Nutr. 133:1158-1162.
Berggren, A (2011) Randomised, double-blind and
placebo-controlled study using new probiotic lactobacilli for strengthening the
body immune defence against viral infections. European Journal of Nutrition 50(3): 203-10.
Cremonini, F., et al (2002) Meta-analysis: the effect of
probiotic administration on antibiotic-associated diarrhoea. Alimentary Pharmacology &
Therapeutics 16 (8): 1461-1467.
de dios Pozo-Olano J, et al (1978). Effect of lactobacilli
preparation on traveler's diarrhea. A randomized, double blind clinical
trial. Gastroenterology. 74:829-30.
Elli M, et al (2006) Survival of Yogurt Bacteria in the Human
Gut. Appl Environ Microbiol 72(7):
5113-7.
Gao X, et al (2010) Dose -- Response Efficacy of a Proprietary
Probiotic Formula of Lactobacillus acidophilus CL1285 and
Lactobacillus casei LBC80R for Antibiotic-Associated Diarrhea and Clostridium
difficile-Associated Diarrhea Prophylaxis in Adult Patients. Am J Gastroenterol. February 9, 2010.
Health Canada monograph on Probiotics, Date: February 11, 2009.
Hempel, et al (2012). Probiotics for the Prevention and
Treatment of Antibiotic-Associated Diarrhea, A Systematic Review and
Meta-analysis. JAMA. 307 (18): 1959-1969.
Herbrecht, et al (2005) Saccharomyces cerevisiae
Fungemia: An Adverse Effect of Saccharomyces boulardii Probiotic
Administration. Clin. Infect. Diseases 2005(40): 1635-1637.
Hickson M, et al (2007) Use of probiotic Lactobacillus preparation
to prevent diarrhoea associated with antibiotics: randomised double blind
placebo controlled trial. BMJ ;335:80.
Hilton E, et al (1997). Efficacy of Lactobacillus GG as a
Diarrheal Preventative in Travelers. J Travel Med. 1997;4:41-3. 6
Katelaris PH, et al (1995). Lactobacilli to prevent
traveler's diarrhea? N Engl J Med. 333(2):1360-1.
Kim HJ, et al, (2003) A randomized controlled trial of a
probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant
irritable bowel syndrome. Aliment Pharmacol Ther. 17(7):895-904.
Kollaritsch H, et al (1993). Prevention of traveler's
diarrhea with Saccharomyces boulardii. Results of a placebo controlled
double-blind study. Fortschr Med. 111:152-6.
Larsen, CN, et al (2006) Dose-response study of probiotic
bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus
paracasei subsp paracasei CRL-341 in healthy young adults. European Journal of Clinical Nutrition 60: 1284-93.
Leyer, L et al (2009) Probiotic Effects on Cold and
Influenza-Like Symptom Incidence and Duration in Children, Pediatrics 124;2 172-179
Marshall-Jones, ZV, et al (2006) Effects of Lactobacillus
acidophilus DSM13241 as a probiotic in healthy adult cats, American Journal of Veterinary
Research 67(6): 1005-1012.
Mater, DD et al (2005). Streptococcus thermophilus and Lactobacillus
delbrueckii subsp. bulgaricus survive gastrointestinal transit of
healthy volunteers consuming yogurt. FEMS Microbiol Lett 250(2): 185-7.
Munoz, et al (2005). Saccharomyces cerevisiae Fungemia: An
Emerging Infectious Disease. Clin. Infect. Diseases 2005(40): 1625-1634.
O'Mahony, L et al. (2005) Lactobacillus and Bifidobacterium in
Irritable Bowel Syndrome: Symptom Responses and Relationship to Cytokine
Profiles. Gastroenterology 2005 (128):541-551.
Osterlund P, et al (2007) Lactobacillus supplementation
for diarrhoea related to chemotherapy of colorectal cancer: a randomised
study. Br J Cancer 2007;97:1028-34.
Reid, Gregor, et al. (2003) Oral use of Lactobacillus
rhamnosus GR-1 and L. fermentum RC-14 signicantly
alters vaginal flora: randomized, placebo-controlled trial in 64 healthy
women. FEMS Immunology and Medical
Microbiology 35 (2003) 131-134.
Romano, C, et al. (2010) Lactobacillus reuteri in children
with functional abdominal pain (FAP). Journal of Paediatrics and Child Health. 2010 Jul 8. [Epub
ahead of print]
Ruggiero, F et al. (2010) A Randomized Controlled Trial
of Lactobacillus GG in Children with Functional Abdominal
Pain. Pediatrics 126(6): e1445-1452.
Saxelin, M, et al. (1991) Dose Response Colonisation of
Faeces after Oral Administration of Lactobacillus casei Strain GG. Microbial Ecology in Health and
Disease 4(4): 209-214.
Whorwell, P, et al (2006) Efficacy of an Encapsulated
Probiotic Bifidobacterium infantis 35624 in Women with
Irritable Bowel Syndrome. Am J Gastroenterol 101:1581-1590.
Information on this site
is provided for informational purposes only. It is not an endorsement of any
product nor is it meant to substitute for the advice provided by physicians or
other healthcare professionals. The information contained herein should not be
used for diagnosing or treating a health problem or disease. Consumers should
inform their healthcare providers of the dietary supplements they take.