Potassium Iodide (KI) and Potassium Iodate (KIO3)
Review Article: Radioprotective Supplements and OTC Medicines
Choose the Best
Supplement to Protect the Thyroid From Radiation. Find
Out Now Which Potassium Iodide or Iodate Supplements Passed Our Tests!
Medically reviewed and
edited by Tod Cooperman, M.D. 
Last Updated![]() : 03/18/2011 | Initially Posted:
06/25/2002
: 03/18/2011 | Initially Posted:
06/25/2002
Recent Reviews
·
Aloe Juices, Gels, and Supplements
Review
·
NAD Booster Supplements Review (NAD+/NADH,
Nicotinamide Riboside, and NMN)
·
PQQ (Pyrroloquinoline Quinone)
Supplements Review
What It Is:
Potassium iodide (KI) and potassium iodate (KIO3) are compounds that can deliver usable,
stable iodine to the body. Both forms can be equally effective but vary
somewhat in taste and dosage (See ConsumerTips: What to
Consider when Buying for more information about the
forms and brands).
What It Does:
As demonstrated following the Chernobyl nuclear reactor accident in 1986,
iodine supplements can protect the thyroid gland from the effects of
radioactive iodine released by such accidents, as well as from other nuclear
events. It is an effective means by which to protect against the effects of
radioactive iodine when evacuation, sheltering, or avoidance of contaminated
food and milk cannot prevent exposure. KI and KIO3 cannot, however, protect against
absorption of materials besides radioactive iodine.
Thyroid cancer rates have been reported to increase by as much as 100 fold in exposed populations, with malignancies beginning
approximately four years after exposure. Children are particularly sensitive to
radioactive iodine because their thyroid glands are very active. In fact,
prophylaxis with stable iodine is recommended for children when even low doses
of radiation are present, while adult prophylaxis is only recommended at higher
levels of exposure. (See ConsumerTips: What to
Consider When Using for more information.)
Radioactive iodine released during a nuclear accident or explosion moves in the
environment through the air and can be inhaled, making it most dangerous to
individuals downwind of the accident or explosion. As it moves, it can also
affect drinking water and exposed crops. Milk from animals grazing on exposed
grasses is also affected and, in the Chernobyl incident, milk exposed many
people to radioactive iodine who were not otherwise directly exposed. Iodine is
needed by the thyroid gland to produce hormones. The iodine pills essentially
saturate the thyroid gland with non-radioactive iodine and block the uptake of
radioactive iodine. Stable iodine is most effective if taken a few hours prior
to exposure but can be beneficial even if taken within three hours after
exposure.
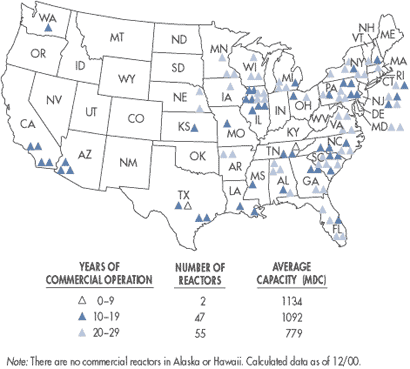
There are more than 100 nuclear reactors operating in the United States. As
shown in the diagram below, states with the highest number of reactors are
Illinois (11), Pennsylvania (9), New York (6), South Carolina (7), Alabama (5),
Florida (5), North Carolina (5), California (4), Georgia (4), Michigan (4), New
Jersey (4) and Texas (4), and Virginia (4).
|
Map of Power Reactor
Sites |
|
|
|
Source: Nuclear Regulatory Commission http://www.nrc.gov/reactors/operating/map-power-reactors.html.
A list of reactors is also available at http://www.nrc.gov/reactors/operating/list-power-reactor-units.html. |
Currently, the Nuclear Regulatory Commission (NRC) offers KI tablets at
no cost to States that request it for people within 10 miles of a
nuclear power plant. However, to date, only 15 of the 35 eligible States have
accepted the NRC's offer or had a pre-existing public KI stockpile, and some
States, such as Illinois and Georgia, are on record as staunchly in opposition
to this simple, effective drug out of concern that people will feel protected
from all nuclear consequences and not evacuate. States that have accepted the
tablets have largely left the distribution up to those municipalities having
residents within 10-mile radius zone of nuclear power plants.
The Public Health Security and Bioterrorism Preparedness and Response Act of
2002 recently passed by Congress includes a provision (introduced by
Congressman Edward J. Markey of Massachusetts) that expands the radius of KI
distribution to twenty miles. However, currently outside the ten-mile radius of
the nuclear facility, the Federal Emergency Management Agency (FEMA) is
responsible for regulating the distribution of KI. The Department of Health and
Human Services controls the national pharmaceutical stockpiles that are to be
sent rapidly into emergencies. And other government agencies control evacuation
of radiological emergency zones. To make matters even more confusing, if you
happen to live within a ten-mile radius of a nuclear weapons facility, the
Department of Energy controls the distribution of the KI. Because of the
potential for bureaucratic delays due to overlapping responsibilities,
President Bush, in his recent proposal to create a cabinet-level Department of
Homeland Security, highlighted KI as an illustrative example of the need for
better coordination among the various federal agencies and State and local
authorities charged with protecting public health in the event of a
radiological emergency. The proposal characterizes KI as a "crucial
drug" and calls for a single agency to be responsible for its
distribution.
Meanwhile, the American Thyroid Association recommends for distribution of
pills to individuals living within 50 miles of a nuclear facility, extra
stockpiles at emergency centers within that radius, and additional stockpiles
at public facilities from 50 to 200-miles out.
Quality Concerns and
Tests Performed:
Some KI products are sold as over-the-counter medications and others, including
KIO3 products, are sold as dietary supplements. While OTC products are required
to be manufactured under more strict guidelines and in FDA approved facilities
than dietary supplements, neither type of product is routinely tested for
quality prior to sale by any government agency. Consequently, ConsumerLab.com
as part of its mission to independently evaluate products that affect health,
wellness, and nutrition, purchased and tested KI and KIO3 products for the
following: (See How Products Were Evaluated for more
information):
·
Labeled Amount Does the product really contain the
labeled amount of stable iodine? Too little iodine in a supplement is a problem
if relying on it to block radioactive iodine. Excessive consumption,
particularly among infants, can affect thyroid functioning and metabolism.
·
Availability for absorption Once in your body, will the pill break
down (disintegrate) properly so that it can be absorbed?
In addition, because
timely administration of stable iodine is crucial during an emergency but
government distribution systems are largely untested at this time,
ConsumerLab.com sought to determine how effectively the products could be
obtained through local pharmacies although not a requirement for a product to
pass testing.
What CL Found:
Getting it:
Checking with various pharmacies across the country near nuclear reactors, CL
found that it was virtually impossible in April and May 2002 to obtain these KI
or KIO3 locally. Many
pharmacies did not have any available and could not order it. Others did not
have any on hand, but said that they could order with a prescription even
though the KI and KIO3 are not
prescription drugs but supplements or over-the-counter medicines. CL was,
however, able to find several brands of KI or KIO3 sold on the Internet, with shipments
taking approximately 4 to 12 days.
After purchasing the products, it came to ConsumerLab.com's
attention that some local pharmacies supposedly do sell KI or KIO3 products and municipalities near nuclear
reactors have begun to list such pharmacies on their Web sites. Interestingly,
an instance of potential price gauging was recently reported in Westchester
Country, New York, where a local pharmacy was charging $75 for 14 pills which
is eight to fourteen times the normal price.
CL purchased six products through the Internet: Three KI tablet products, one
KI powder product, and two KIO3 tablet products. Two of the KI tablet products are
approved by the FDA as over-the-counter (OTC) medications, indicating that they
produced under more rigid quality controls than products sold as supplements.
Of the six products, all were found to contain the labeled amount of KI or KIO3. In addition, all tablet forms were found to
disintegrate properly in solution, indicating that they would be available for
absorption. These results compare very favorably to other tests conducted by
ConsumerLab.com on other mineral, vitamin, and herbal supplements.
Products That Passed
Testing:
Listed below, alphabetically by name are the products that passed ConsumerLab.com's independent testing, as well as their
prices, and the Web sites and phone numbers for companies from which products
were purchased.
|
CONSUMERLAB.COM APPROVED QUALITY POTASSIUM IODIDE (KI) and
POTASSIUM IODATE (KIO3) PRODUCTS |
||||
|
Product Name, Amount
of Potassium Iodide/Iodate per Unit |
Manufacturer or
Distributor |
Cost per dose (130 mg
of KI or 170 mg of KIO3) |
Retail Price (excludes |
Where Purchased |
|
Potassium Iodide (KI): |
||||
|
Iosat® (Potassium Iodide Tablets, U.S.P.)
(130mg/tablet)1 |
Dist by NukePills.com |
$0.66 |
$9.25 |
Nukepills.com |
|
Potassium Iodide U.S.P. Granular (1 lb. of KI
bulk) |
Dist by Ruger Chemical Co., Inc. |
$0.02 |
$40.49 |
Medichest.com |
|
RAD BLOCK KI Radiation Blocking Tablets (65
mg/tablet) |
Dist by USDPI |
$0.20 |
$19.95 |
ApprovedGas |
|
THYRO-BLOCK® potassium iodide tablets, USP
(130mg/tablet)1 |
Dist by Wallace Laboratories |
$0.61 |
$58.97 |
Nitro-pak.com |
|
Potassium Iodate (KI03): |
||||
|
KI4U Thyroid Blocking Tablets KIO3 (85 mg/tablet) |
Dist by KI4U, Inc. |
$0.48 |
$48.00 |
KI4U.com |
|
Medical Corps Potassium Iodate (85mg/ tablet) |
Dist by Medical Corps |
$0.18 |
$18.00 |
Baproducts.com |
|
1 Registered
with the FDA as an over-the-counter (OTC) medicine, as opposed to a dietary
supplement. |
||||
|
Unless otherwise noted, information about the products
listed above is based on the samples purchased by ConsumerLab.com (CL) for
this Product Review. Manufacturers may change ingredients and label
information at any time, so be sure to check labels carefully when evaluating
the products you use or buy. If a product's
ingredients differ from what is listed above, it may not necessarily be of
the same quality as what was tested. |
||||
|
Copyright ConsumerLab.com, LLC, 2002. All rights reserved.
Not to be reproduced, excerpted, linked to, or cited in any fashion without
the express written permission of ConsumerLab.com LLC. |
||||
It cannot be assumed that other products from a manufacturer or distributor
listed above are of similar quality to those that passed testing. If a specific
product is not listed, it either has not passed testing or was not tested.
(Note to manufacturers: Testing of additional products may be requested through
ConsumerLab.com's Voluntary Testing Program. Products that
pass this testing will be added to the list above.)
ConsumerTips:
What to
Consider When Buying:
There is no decisive difference in bioavailability or shelf life between
potassium iodide (KI) and potassium iodate (KIO3). Potassium iodide (KI) has a very bitter
taste, while KIO3 does not, which may
make KIO3 preferable if pills
need to be crushed into smaller doses for children. To mask the bitter taste,
the Thyro-Block® KI product has a coating; the Iosat®
KI product does not, although it is a very small pill. If being used for
children, you may want to consider that some of the products have a lower dose
per pill, reducing the amount of pill splitting for children's doses.
Bulk powder products are more economical than tablets since a
single bottle can create hundreds or even thousands of doses for only pennies a
dose, but are not generally recommended because they can cause
irritation to skin, eyes, and the respiratory tract during handling as well as
requiring equipment for measurement and a way to deliver the medicine
such as a liquid to make a solution.
Only three products, Iosat®, ThyroSafe®,
and ThyroShield® are currently registered with the
FDA as over-the-counter medications, indicating that they are manufactured
under stricter regulations than the other products sold as dietary supplements.
(THYRO-BLOCK was also FDA registered but has been discontinued.) It should also
be noted that, according to the FDA,
"only the KI products approved by the FDA may be legally marketed in the
United States." However, all of the products tested in this review were
found to be of comparable quality in terms of the parameters tested.
It is recommended that all stable iodine products be kept in a tightly sealed
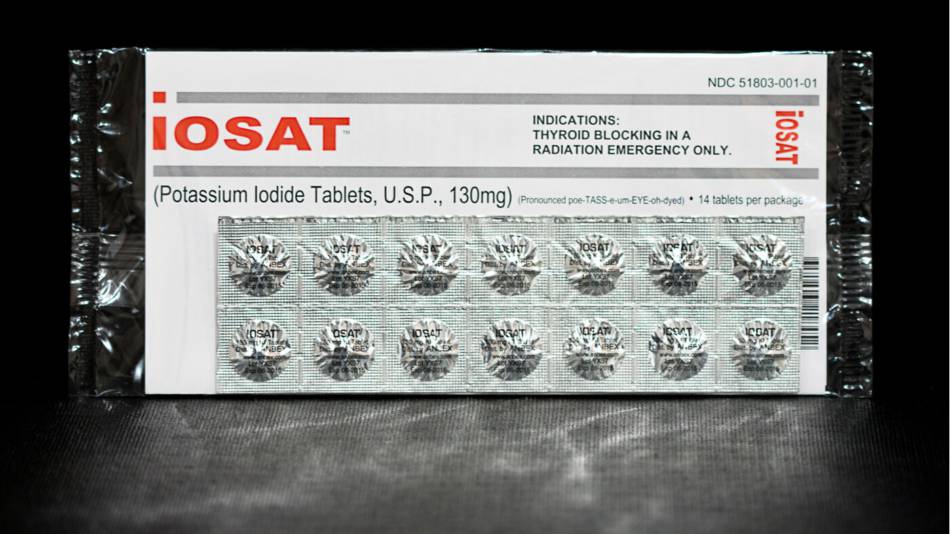
container away from exposure to light or extreme temperatures. The Iosat® product comes in a blister pack with each tablet
individually sealed. This may help reduce degradation and allows for ease
distribution of individual tablets, which is why it is the product purchased by
the Nuclear Regulatory Commission (NRC) for distribution to states.
Tincture of iodine contains elemental iodine, not stable iodine, and cannot be
used as a radioprotective agent. Iodized salt does contain stable iodine, but
in an amount so small that a sufficient dose could not be realistically
consumed several hundred teaspoonsful of iodized
salt would be needed for a single dose. Also, there are iodine supplements on
the market designed to prevent iodine deficiency, but contain far less stable
iodine than required for protecting against radioactive iodine. Such
supplements typically contain only a few hundred micrograms (mcg)
of potassium iodide or potassium iodate, which is several hundred times smaller
in dose than the milligram (mg) doses needed for protection from a
nuclear event.
What to Consider When Using:
KI or KIO3 is only necessary
when exposure to radioactive iodine exceeds certain thresholds (expressed in
"rems" or "centigrays" (cGys), which are equivalent). As shown in the table below,
the threshold levels are much lower for children and pregnant or nursing
mothers than for other adults. The recommended dose also decreases as age
decreases.
|
Threshold Thyroid
Radioactive Exposures and Recommended Doses of KI for Different Risk Groups |
||||
|
Risk Group |
Predicted Thyroid
exposure (cGy) |
KI dose (mg) |
# of
130 mg tablets |
# of
65 mg tablets |
|
Adults over 40 yrs |
≥500 |
130 |
1 |
2 |
|
Adults over 18 through
40 yrs |
≥10 |
130 |
1 |
2 |
|
Pregnant or lactating
women |
≥5 |
130 |
1 |
2 |
|
Adolescents over 12
through 18 yrs* |
≥5 |
65 |
1/2 |
1 |
|
Children over 3
through 12 yrs |
≥5 |
65 |
1/2 |
1 |
|
Over 1 month through 3
years |
≥5 |
32 |
1/4 |
1/2 |
|
Birth through 1 month |
≥5 |
16 |
1/8 |
1/4 |
|
* Adolescents
approaching adult size (≥ 70 kg) should receive the full adult dose
(130 mg). |
||||
|
Source: U.S. Food and Drug Administration, Center for Drug
Evaluation and Research, 2001, Guidance: Potassium Iodide as a
Thyroid Blocking Agent in Radiation Emergencies. |
||||
Seventy-six (76.45%) percent of KI is iodine. That means that a 130 mg dose of
KI contains about 99.4 mg of iodine. However 59.3% of
KIO3 is iodine.
Consequently, doses of KIO3 need to be 34% higher than those shown above for KI to
provide an equivalent amount (99.1 mg) of iodine. For example, an adult daily
dose of KIO3 would be about 170
mg (most KIO3 products come as
170 mg or 85 mg (half dose) tablets).
The protective effect of stable iodine lasts approximately 24 hours, so it
should be taken daily until a risk of significant exposure to radioactive
iodine inhalation or ingestion no longer exists. However, repeat dosing of
infants less than one month of age is not recommended due to safety concerns
(see Concerns and Cautions). As a powder or uncoated tablet, KI has a very
bitter taste. Stable iodine from crushed tablets may be diluted in milk,
formula, or water and the appropriate volume administered to babies. It has
also been suggested that tablets or parts of tablets can be encased in a small
amount of bread or other food and swallowed. Pregnant women should be given
stable iodine for their own protection and for that of the fetus. However,
because of the risk of blocking fetal thyroid function, repeat dosing (more
than one or two doses) of pregnant women should be avoided. Women who are
breastfeeding should be administered stable iodine for their own protection and
to potentially reduce the radioactive iodine content of the breast milk, but
not as a means to deliver iodine to infants, who should get their KI or KIO3 directly. Repeat dosing should be
avoided in the lactating mother, except during severe contamination, as too
much in breast milk may also pose a risk of hypothyroidism in nursing neonates.
Once the radioactive plume has passed, further radiation protection is ideally
accomplished by food control measures and not by repeated administration of
stable iodine. Because of radioactive decay, foods stored for weeks to months
after production pose no radiation risk.
Concerns and Cautions:
The risks
of stable iodine administration include inflammation of the salivary gland,
gastrointestinal disturbances, allergic reactions and minor rashes. In
addition, persons with known iodine sensitivity should avoid KI and KIO3, as should individuals with dermatitis
herpetiformis and hypocomplementemic vasculitis,
extremely rare conditions associated with an increased risk of iodine
hypersensitivity.
Thyroidal side effects of stable iodine include iodine-induced thryotoxicosis, which is more common in older people and in
iodine deficient geographic areas but usually requires repeated doses of stable
iodine. In addition, iodide goiter and hypothyroidism are potential side
effects more common in iodine sufficient areas, but they require chronic high
doses of stable iodine. Individuals with multinodular goiter, Graves' disease,
autoimmune thryroiditis should be treated with
caution, especially if dosing extends beyond a few days. The vast majority of
such individuals will be adults.
A small percentage of infants under one month of age may experience transient
hypothyroidism, which can impair intellectual development. However, the
benefits of treatment outweigh this risk. It is recommended that neonates
treated with KI be monitored for this effect and that thyroid hormone therapy
be instituted in cases in which hypothyroidism develops.
For additional information, see the FDA's Frequently Asked Questions on
Potassium Iodide (KI).
Information on this site
is provided for informational purposes only. It is not an endorsement of any
product nor is it meant to substitute for the advice provided by physicians or
other healthcare professionals. The information contained herein should not be
used for diagnosing or treating a health problem or disease. Consumers should
inform their healthcare providers of the dietary supplements they take.
Related CL Answers (2)