Echinacea Supplements Review
Many Echinacea
Supplements Fail CL's Tests. Make Sure You Know What You're Getting!
Medically reviewed and
edited by Tod Cooperman, M.D. 
Last Updated![]() : 07/17/2021 | Initially Posted:
02/06/2021
: 07/17/2021 | Initially Posted:
02/06/2021

Recent Reviews
·
Aloe Juices, Gels, and Supplements
Review
·
NAD Booster Supplements Review
(NAD+/NADH, Nicotinamide Riboside, and NMN)
·
PQQ (Pyrroloquinoline Quinone)
Supplements Review
Table of Contents
Summary
·
What do Echinacea supplements do? Echinacea
supplements may modestly help prevent colds when taken during cold season
and/or reduce the severity of a cold if taken at the first onset of symptoms —
although the evidence is mixed. Limited evidence suggests that a form of
Echinacea may modestly reduce anxiety (See What It Does). Phenolic compounds in Echinacea
may be responsible for some of the herb's effects (See What It Is).
·
What did CL's tests of Echinacea find? Four products did
not contain the expected minimum amounts of total phenolic compounds
(one had less than 2%), and two products did not even list their amounts of
Echinacea (See What CL Found).
·
Which Echinacea supplements are best? Among six products
Approved for Quality by ConsumerLab.com, two (an extract and a non-extract)
were chosen as Top Picks.
·
Which form and dose of Echinacea is best? Echinacea comes in
various forms and concentrations, but most positive clinical studies used
products extracted from aerial parts (leaves, flowers, stems) of E.
purpurea or the roots of E. angustifolia (See ConsumerTips™).
·
Is Echinacea safe? Echinacea appears to be relatively safe
for short-term use (up to 12 weeks), and side-effects tend to be mild (GI
symptoms, increases urination). However, be aware that some people may have
allergic reactions, such as rashes, and rarely, liver damage has been reported.
Although Echinacea may affect the metabolism of a variety of drugs, the effect
may be small (See Concerns and Cautions).
What It Is:
Echinacea
— commonly known as purple coneflower — is a group of nine related plant
species indigenous to North America. Three species of Echinacea — Echinacea
purpurea, E. angustifolia, and E. pallida —
have been used medicinally and are commonly found in dietary supplements.
Supplements can be made from the above-ground herb (aerial) and/or root
portions of the plant, or a combination of both.
Compounds known as phenols (including cichoric, caftaric, and chlorogenic acids as well as echinacoside and cynarin) are
known to exist in Echinacea and have been shown to inhibit steps in the
development of inflammation. Cichoric acid
(alternatively spelled "chicoric" acid),
also shows immunostimulatory properties (Hudson, Pharmaceuticals (Basel)
2011).
Laboratory tests show that cichoric acid and other
compounds from Echinacea purpurea aerial parts may inhibit the
replication of certain viruses, including HSV-1 and -2, respiratory syncytial
virus, rhinoviruses, and coronaviruses (Hudson, Pharmaceuticals (Basel) 2011).
According to the United State Pharmacopeia (USP), the phenols in dried aerial
parts of E. purpurea make up at least 1% of the dried herb
(powder), which would be about 0.25% in fresh herb (since it's about 75%
water). Most of this will be cichoric acid, followed
by caftaric acid. In E. purpurea root,
caftaric, cichoric and
chlorogenic acids make up at least 0.5% of the dried root.
In E. angustifolia root, the dominant phenols are echinacoside, cynarin, and
chlorogenic acid, constituting 0.5% of dried root. In E. pallida root, the
phenols are caftaric, cichoric,
and chlorogenic acids as well as echinacoside and
these make up 0.5% of dried root.
Other constituents that may be important in the effects of Echinacea include alkamides (such as isobutylamides),
which occur at very low concentrations (0.01%) in dried E. purpurea aerial
parts but at a higher concentration (0.75%) in E. angustifolia root, as well as
polysaccharides (which are also found in other botanical ingredients as well as
in grains, vegetables, fruit, nuts, and cereal bran) but are generally removed
from extracts.
Product lists as extracts typically contain about four times the concentration
of phenols expected in dried herb or root, but this can vary.
What It Does:
For colds:
Echinacea's popularity is primarily due to its reported ability to reduce the
symptoms and duration of colds and flu-like illnesses. It is believed to work
through modulation of the immune system. The German Commission E, World Health
Organization (WHO) and Canadian Natural Health Products Directorate have all
advocated the use of Echinacea for upper respiratory tract symptoms related to
the common cold; however the results of clinical
trials have been mixed.
A difficulty in comparing the results of clinical trials (and perhaps the
reason for varying results) with Echinacea is that studies use varying doses,
species and parts of the Echinacea plant. The different species and plant parts
used can contain different concentrations of compounds, and even the same
plant, like all plants, may contain differing levels of these compounds at
different times of the year or when grown in different geographical locations (Qu, HortScience 2005).
It is yet to be determined if it is one, a few, or the combined effect of many
constituents that are responsible for Echinacea's immune-modulating properties.
Another difficulty is that studies employ Echinacea at varying times in the
course of a cold: prophylactically before a cold begins; at the first symptoms
of a cold; and/or several weeks after a cold, all of which could impact the
herb's effectiveness.
In 2007, a meta-analysis (in which results of many clinical trials are pooled
and analyzed) showed prophylactic use of Echinacea reduced the incidence of
colds by 65%, but by only 35% if taken at the first sign of symptoms (Shah, Lancet Infect Dis 2007).
On the other hand, a 2008 report showed that taking an E. purpurea extract
during the winter months did not significantly prevent the onset of upper
respiratory tract infections versus placebo (O'Neil, Ann Allergy Asthma Immunol 2008). This
study, however, did not list the part of the Echinacea plant used nor did it
indicate analytical work to confirm the contents. A large controlled trial (Barrett, Ann Int Med 2010) giving a five-day
course of Echinacea at the first sign of symptoms failed to show a substantial
benefit. That study used tablets made by MediHerb
(distributed in the U.S. as Echinacea Premium by Standard Process) containing
dried extracts of E. purpurea and E. angustifolia root.
A well-controlled study in which ethanolic extracts of E. angustifolia root
and E. purpurea root were compared to placebo showed no
significant benefit for either Echinacea preparation in preventing upper
respiratory tract infections relative to placebo, despite participants in the
Echinacea groups believing that they had more benefit than those in the placebo
group. Adverse effects were much higher in the E. angustifolia group
compared to placebo group (Melchart, Arch Fam Med
1998).
A placebo-controlled study among children in the Seattle, Washington area found
no benefit in reducing the duration or symptoms of colds when a non-alcoholic
preparation of pressed juice from E. purpurea aerial parts
(apparently provided by Madaus, AG, Germany) was
taken twice a day starting at the onset of symptoms and continued throughout
the upper respiratory infection for a maximum of 10 days. Rash occurred in 7.1%
of the children given Echinacea versus 2.7% of those given placebo (Taylor, JAMA, 2003).
The largest study of Echinacea suggests it to be
modestly effective at preventing colds if taken three times daily throughout
the cold season (and five times daily during a cold) (Jawad, Evid Based Compl Alt Med
2012). Compared to those taking placebo, the Echinacea-treated group
experienced 26% fewer cold "events" (colds and days spent with colds,
combined). In addition, there were fewer recurrent colds among those treated
and they took less pain medication, such as aspirin, during colds. The study
used a liquid supplement (Echinaforce from
A. Vogel Bioforce AG, which is among the products
tested in the review below) made from freshly harvested E. purpurea (95%
aerial parts and 5% root) as alcoholic extract. Participants in the study
swallowed a 0.9 mL (33 drops) dose three times per day, and, during acute
stages of a cold this increased to five times per day. Oddly, the use
instructions on Echinaforce sold in
the U.S. when we tested it in 2016 suggested a smaller dose -- only 20 to 25
drops (about 0.6 mL) three times a day, and, when tested for the current
review, the label suggested an even lower dose of just 15 drops two to three
times per day, raising a question of how effective these lower doses may be. In
the study, drops of Echinaforce were
diluted in water and retained in the mouth for 10 seconds before being
swallowed "to provide maximum local antiviral effects," according to
the study, although it interesting to note that Echinaforce is
65% ethanol, which, itself, may have direct antiviral properties. Most people
were not 100% compliant with the treatment, but those who were experienced even
greater reductions in colds.
Several small, company-funded studies suggest that taking
6 tablets of Esberitox (distributed
in the U.S. by Nature's Way and sold in Europe as Esberitox
N) three times daily during a cold or respiratory tract infection may
hasten improvements in symptoms (i.e. nasal congestion, hoarseness, chest pain)
by about one to three days -- especially when taken at the earliest onset of
symptoms (Zepelin, Curr Med Res Opin 1999; Naser, Phytomedicine 2005). Among adults with
acute exacerbation of chronic bronchitis who took an antibiotic, those who also
took liquid Esberitox N drops
three times daily (a total daily dose of 8.8 mL) for 28 days were breathing
slightly better at day 10 of treatment than those given placebo (Hauke, Chemotherapy 2002). Esberitox is
a proprietary blend of extracts of E. purpurea and E.
pallida root, wild indigo (Baptisia tinctorial) root, and Thuja
occidentalis leaf (an ingredient that may potentially cause seizures
in people with seizure disorder) (Millet, Clin Toxicol 1981).
No serious adverse events were reported in these studies; mild to moderate side
effects such nausea and insomnia were reported by a small number of
participants.
An alcoholic extract of the root portion of E. pallida (900 mg
per day) showed some promise relative to a placebo in reducing the average
length of a cold (Dorn, Complement Ther Med 1997).
For anxiety:
Although it's been proposed that echinacea may have anti-anxiety effects, the
evidence is weak and conflicting. Two small studies (by the same research
group) showed that an E. angustifolia root extract (Anxiofit-1 from Anxiofit Ltd., standardized to 3% echinoacoside
and containing 1-1.5% alkamides — which may be
responsible for any anxiolytic effects) decreased anxiety scores in patients
with subthreshold/mild anxiety and generalized anxiety disorder. However, a
panel of the European Foods Safety Authority (EFSA) that reviewed these studies
considered them insufficient to establish a cause and effect relationship and,
therefore, refused to grant the product the right to claim that it could reduce
subthreshold and mild anxiety (EFSA J, 2016).
A later placebo-controlled clinical study of Anxiofit-1
(marketed in the U.S. as AnxioCalm) given at a dose
of 40 mg three times daily for seven days showed it to modestly reduce levels
of anxiety in response to anxiety-producing situations but not underlying
anxiety. The lead researcher holds a patent on the anxiolytic effects of
Echinacea (Haller, Phytother
Res 2020). A more recent study
in which the same extract was given at a dose of 20 mg or 40 mg twice daily
(total daily dosage 40 mg or 80 mg) to 93 men and women with mild to moderate
anxiety for six weeks showed very modest improvements in self-reported moods
and emotions, but no reduction in anxiety compared to placebo. The study was
funded by EuroPharma Inc., a U.S. distributor of AxioCalm (Lopresti, J Affect Disord 2021).
Coronavirus effects:
An in-vitro study found Echinaforce (A.
Vogel AG) inhibited specific common cold coronaviruses and SARS-CoV-2, the
coronavirus that causes COVID-19.
The study was funded by the maker of Echinaforce and
authored, in part, by two A. Vogel employees as well as recipient of a grant
from that company (Signer, Virology 2020).
It is interesting to note that Echinaforce is
65% ethyl alcohol (ethanol) which, itself, can inhibit viruses, but, the
researchers explained that they had extensively diluted Echinaforce in the experiment and showed that
similarly diluted ethyl alcohol alone would not cause viral inhibition (Signer, Virology 2020 — author correction). There
is no clinical evidence that taking this or any other echinacea product
can prevent or treat coronavirus
infections in people.
See ConsumerTips™ for
information about more dosing and usage.
Quality Concerns and
Tests Performed:
Neither
the U.S. Food and Drug Administration (FDA), nor any other federal or state
agency, routinely tests Echinacea products, nor any supplement, for quality
prior to sale. A quality concern with herbal supplements is potential
contamination with heavy metals such as lead, cadmium and arsenic. Even if
ingested at low levels, heavy metals can accumulate and cause damage over time.
Cadmium, for example, builds up in kidneys and, if it reaches a high enough
level, can cause kidney damage. Lead, which accumulates in bones, can cause a
variety of symptoms including abdominal pain, impaired mental functioning, high
blood pressure and anemia. Another concern with herb and root powders is
contamination with Escherichia coli, Salmonella spp.,
yeast and mold that can occur during growing, harvesting or preparation as a
supplement. As a result, the WHO has established standards for Salmonella contamination
of medicinal plant materials intended for internal use. Furthermore the U.S.
Food and Drug Administration (FDA) has established zero tolerance levels
for Salmonella.
ConsumerLab.com, as part of its mission to independently evaluate products that
affect health, wellness, and nutrition, purchased many E. purpurea and E.
angustifolia supplements sold in the U.S. and tested all of them to
determine whether they 1) possessed the claimed and minimum expected amount of
phenols [link to What It Is section] (shown in our results as total phenolic
compounds or TPCs), 2) if products containing herb or root powders or at least
250 mg of minerals per suggested daily serving were free of unacceptable levels
of lead, cadmium, and arsenic, 3) if products containing herb or root powders
were free of contamination with Escherichia coli, Salmonella
spp., yeast and mold, and 4) all regular tablets and caplets would properly
disintegrate to enable absorption of their contents (see Testing Methods and Passing Score).
What CL Found:
It's not easy to compare Echinacea products, even after
analyzing them in a laboratory as we have done, because the ideal dose of
Echinacea, as well as form and delivery, is not well defined. However,
chemically, we can get an idea of how much Echinacea is in each product by
looking for total phenolic compounds (TPCs) and, from a safety perspective, we
can look at how free each product is from common contaminants. Contamination:
All products made from whole parts of Echinacea (as opposed to an extract) were
tested for heavy metal contamination and pathogenic microbes (yeast,
mold, Salmonella, and E. coli.). None of the products
exceeded contamination limits, which is an improvement over our report in 2016
in which one product was contaminated with 2.2 mcg of lead per daily serving.
Amount of Echinacea — Based on Phenols Found:
To try to assess the amount of Echinacea in each product, ConsumerLab.com
tested for total phenolic compounds (TPCs) known to exist at certain levels in
various forms of Echinacea, allowing ConsumerLab.com to set minimum
expectations and compare products. These amounts are shown in the both the
graph below and results table further below.
As you can see in the graph, the amount of TPCs per suggested serving ranged
across products from as much as 50.9 mg to as little as 0.041 mg (over a
120,000% difference!).
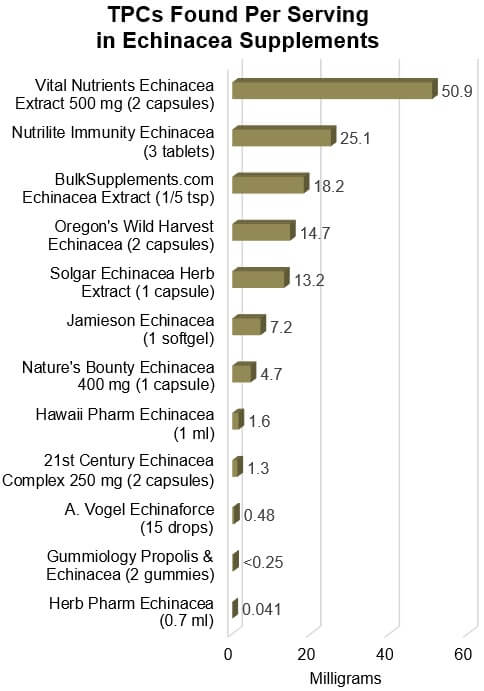
Four products were "Not Approved" because they
failed to contain the minimum amounts of TPCs we would expect based on their
claimed amounts and types of Echinacea:
·
Gummiology Propolis & Echinacea claimed to contain
400 mg of E. purpurea herb (aerial) extract, from which we
would expect a minimum of 16 mg of TPCs, but we found less than 0.25 mg — less
than 2% of what we expected.
·
Herb Pharm Echinacea claimed to contain the equivalent of 280
mg of fresh E. purpurea root per 0.7 ml serving (which, we
assume was one dropperful, but was not noted), from which we would expect 0.7
mg of TPCs, but we found only 0.041 mg — just 6% of what we expected.
·
Jamieson Echinacea claimed 400 mg of E.
purpurea tops (aerial) extract equivalent to 4,000 mg of raw herb, from
which we would expect 10 mg of TPCs, but we found 7.2 mg, or 72% of what we
expected, although this was a higher amount of TPCs than the majority of
products.
·
21st Century Echinacea claimed 250 mg of a blend of E.
purpurea herb (aerial) extract and E. angustifolia root
extract. When blends are listed, they must be in order of greatest to smallest,
so the majority of the blend should be E. purpurea, and we would
expect about 5.8 mg to 9.9 mg of TPCs per two-capsule serving. However, we
found only 1.3 mg — only about 13% to 22% of what we expected.
What is also striking
about these results is that the most clinically studied product, A. Vogel Echinaforce, provides a very small amount of TPCs,
indicating that it is made from a very small amount of Echinacea. It does not
even list an amount of Echinacea on the label -- which is why were not able to
consider an approval status for it.
Interestingly, when we tested Echinaforce in
2016, its label was more descriptive than it is now: It claimed to be made from
521 mg of fresh E. purpurea herb and 27 mg of fresh E.
angustifolia root per 20 drop serving (to be given three times per
day). In 20 drops of that product, we found just 0.18 mg of TPCs, although we
expected much more (1.37 mg) based on the fresh Echinacea it claimed, so it was
Not Approved. Our recent tests of the newer product show that a serving (now
listed as 15 drops) contained a higher amount of TPCs (0.48 mg) than
previously, although still a relatively small amount. It's odd that the
suggested serving size has changed since 2016 (as has the number of serving per
day, which is now two to three, rather than three) and from the original 33
drops three times per day used in a published study -- Jawad, Evid Based Compl Alt Med
2012).
Cost
To provide a rough price comparison, we calculated how much you would need to
pay to get an equivalent amount of TPCs (10 mg) from each product based on the
amount of TPCs found. This cost ranged from just 4 cents to
$67.97.
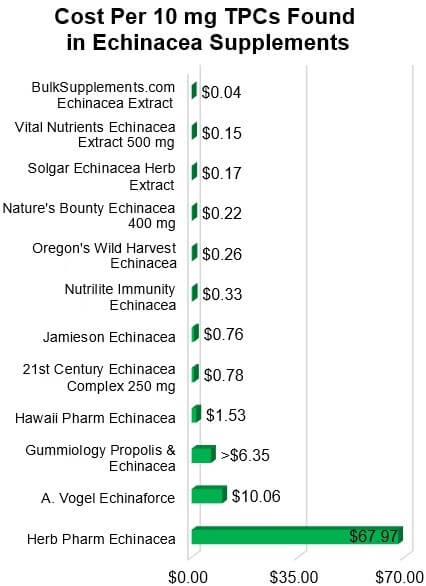
Top Picks:
Due to
a lack of consistent clinical information (see What It Does), it's not possible to say with
confidence that any Echinacea product will be beneficial in preventing or
treating colds, flu, or other condition. Nevertheless, should you wish to use
Echinacea, those which we believe deliver the best quality and are, therefore,
our Top Picks among Approved products are the following, by
category:
E. purpurea herb extract:
Vital Nutrients Echinacea Extract 500 mg is our Top Pick among E.
purpurea herb extracts, as it provides the largest amount of TPCs
(50.9 mg) per serving (i.e., 2 capsules, providing 1,000 mg of extract).
Although somewhat expensive (79 cents per serving), it is less expensive in
terms of TPCs than any other product aside from BulkSupplements.com, a loose
powder that we found tasted somewhat burnt (although it contained its expected
TPCs). Several clinical trials in which Echinacea has shown benefit in preventing/treating
colds used extracts (around 900 mg per day).
Although tinctures, such as Echinaforce,
made from primarily the (aerial) above-ground plant parts of E.
purpurea, have shown benefits in clinical trials, we are concerned about
the low amounts of TPCs in Echinaforce as
well as the variations we have seen over time in these levels and in its usage
information. We would also prefer that the product state the amount of extract
it is supposed to contain. For these reasons, despite the amount
of studies indicating a benefit (funded by its manufacturer), this product is
neither Approved nor a Top Pick.
E. purpurea herb + E. purpurea and E. angustifolia root powder"
Although there is not much clinical evidence for non-extracts, if you prefer a
non-extract, our Top Pick for a mixture of dried aerial and
root portions of E. purpurea with some E. angustifolia root
is Oregon's Wild Harvest. It blew way past our expected
minimum amount of TPCs (5.7 mg) by delivering 14.7 mg per two-capsule serving
(26 cents), indicating herb powder that is rich in TPCs. If you only want E.
purpurea herb powder, we suggest Nature's Bounty Echinacea 400,
which provides 4.7 mg of TPCs per capsule for only 10 cents. Both of these
products passed our testing for contaminants (heavy metals and microbes), to
which all dried herb/root products were subjected.
Test Results by Product:
Listed
alphabetically below are test results for twelve products. Ten were selected
for testing by ConsumerLab.com and two others (each indicated with CL flask
icon) are included for having passed the same testing through ConsumerLab.com's voluntary Quality Certification Program.
Shown for each product are the claimed amount and form of Echinacea in each
serving and daily serving information from the label. Amounts of total phenolic
compounds (TPCs) expected and found, special features, price, and the cost to
obtain 10 mg of TPCs are also shown. To be listed as "Approved,"
products had to contain their expected amounts of TPCs and, if made from whole
herb (as opposed to extract) had to pass tests for lead, cadmium, and arsenic,
as well as for yeast, mold, Salmonella and E. coli.
The full list of ingredients is available for each in the final column.
Results of
ConsumerLab.com Testing of Echinacea Supplements
(Click arrows or swipe left or right to see all columns)
Product Name
(Suggested Serving on Label)
Echinacea Claimed Per Servingⓘ
TPCs Expected & Found
Heavy Metalⓘ &
Microbial Contaminationⓘ
Suggested Daily Servings
Formⓘ
Cost for Suggested Serving
[Cost Per 10 mg of TPCs Found]
Price
Notable Features
Full List of Ingredients Per Serving
E. purpurea only Products:
A. Vogel Echinaforce®
Dist. by Bioforce (UK)
Ltd
15 drops
Tinctures of fresh E. purpurea herb 95% and fresh E. purpurea root
5%. No amounts listed.
TPCs:
Expected Min: NAⓘ
TPCs: 0.48 mg
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Adults: 15 drops in a little water 2-3 times daily. Children (6-12 yrs): 7 drops in a little water 2-3 times daily.
Liquid from bottle
$0.48/15 drops
[$10.06]
$37.99/50 ml bottle (approx. 79 servings)
Organically grown.
Caution from ConsumerLab: This tincture is 65%
ethanol (grain alcohol).
Ingredients: Tinctures of fresh Echinacea purpurea (Purple
Coneflower) herb 95% and fresh Echinacea purpurea (Purple
Coneflower) root 5%, extracted in ethanol (65% V/V).
APPROVED
BulkSupplements.com Echinacea Extract
Dist. by BulkSupplements.com
1/5 tsp [0.5 g]
500 mg purpurea aerial extract
TPCs:
Claimed: 20 mg
TPCs: 18.2 mg ✔
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Take 500 mg (1/5 tsp) once or twice daily or as directed by a physician.
Powder in pouch
$0.06 per 1/5 tsp
[$0.04]
$12.96/3.5 oz [100 g] pouch (approx. 200 servings)
Free of: Added Sugar, Soy, Dairy, Yeast, Gluten,
Additives.
1/5 tsp
Echinacea Extract (Echinacea purpurea) (Aerial part) Standardized to
contain ≥4% Polyphenols) 500 mg.
Other Ingredients: None.
NOT APPROVED
Gummiology® Propolis & Echinacea
- Raspberry Flavor
2 gummies
400 mg purpurea herb extract
TPCs:
Expected Min: 16 mg
TPCs: <0.25 mg
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Take 2 gummies daily.
Medium/large star-shaped gummy
$0.16/2 gummies
[>$6.35]
$8.00/100 gummies
2 gummies
Vitamin C 70 mg, elderberry extract 200 mg, propolis extract 100 mg
This product is not manufactured with milk, eggs, fish, crustacean
shellfish, tree nuts, peanuts, wheat, soy or gluten.
2 gummies
Calories 15, Total Carbohydrates 4 g, Total Sugars [Includes 4 g Added Sugars]
4 g, Vitamin C (as Ascorbic Acid) 70 mg, Echinacea Extract (Echinacea
purpurea) (Herb) 400 mg, Elderberry Extract (Sambucus nigra) (Herb)
200 mg, more...
NOT APPROVED
Herb Pharm® Echinacea
Dist. by Herb Pharm
0.7 ml
Extract from 280 mg of fresh E. purpurea root (root)
TPCs:
Expected Min: 0.7 mg
TPCs: 0.041 mg
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Add 1 full squeeze of the dropper bulb to 2 oz. of water or juice, 2 to 5 times
per day.
Liquid from bottle
$0.28/0.7 ml
[$67.97]
$11.59/1 fl oz [30 ml] bottle (approx. 42 servings)
Certified Organic. Free (undried). Gluten-Free.
0.7 ml
Echinacea root (Echinacea purpurea) extract 814 mg. Extraction rate 280
mg fresh herb per 0.7 ml.
Other Ingredients: Certified organic vegetable glycerin & distilled water.
NOT APPROVED
Dist. by Jamieson Laboratories
1 softgel
400 mg purpurea herb top (aerial) extract equivalent to 4,000 mg of raw herb
TPCs:
Expected Min: 10 mgⓘ
TPCs: 7.2 mg
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Take 1 softgel daily, at first sight of infection.
Medium/large softgel
$0.55/softgel
[$0.76]
$33.00/60 softgels
Non-GMO. No Starch, gluten, lactose, artificial
colors, flavours or preservatives. No Animal Testing.
Certified Authentic Tru-ID® seal. Helps fight
colds and flu and shorten their duration: may be a drug claim
1 softgel
Echinacea (10:1) extract (Echinacea purpurea, herb top) [equivalent to
4,000 mg of raw herb] 400 mg.
Also contains: Soybean oil, glyceryl palmitostearate, polyglycerol esters of
fatty acids, more...
APPROVED
Nature's Bounty® Echinacea 400 mg![]()
Mfd. by Nature's Bounty, Inc.
1 capsule
400 mg purpurea aerial powder
TPCs:
Expected Min: 4 mg
TPCs: 4.7 mg ✔
Heavy Metals: Pass
Microbial Contamination: Pass
For adults, take one (1) capsule seven times daily, preferably with meals.
Large capsule
$0.10/capsule
[$0.22]
$10.49/100 capsules
Non-GMO. No Artificial Color, No Artificial
Flavor, No Artificial Sweetener, No Preservatives, No Sugar, No Starch, No
Milk, No Lactose, No Soy, No Gluten, No Wheat, No Yeast, No Fish. Sodium Free.
1 capsule
Echinacea (Echinacea purpurea) (aerial) 400 mg.
Other Ingredients: Gelatin.
APPROVED
Top Pick
for E. purpurea herb extracts
Vital Nutrients Echinacea Extract 500 mg
Mfd. by Vital Nutrients
2 vegetarian capsules
1,000 mg purpurea herb extract
TPCs:
Expected Min: 40 mg
TPCs: 50.9 mg ✔
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
1 to 2 capsules 2 to 4 times daily, or as directed by your healthcare
professional.
Large vegetarian capsule
$0.79/2 vegetarian capsules
[$0.15]
$23.60/60 vegetarian capsules
Excludes: Coatings, Binders, Gluten (Wheat, Rye,
Barley), Milk/Dairy (Casein, Whey), Soy Protein, Egg Protein, Sugar.
2 vegetarian capsules
Echinacea purpurea Herb Extract (3-4% phenolics) 1,000 mg.
Other Ingredients: Vegetable Cellulose Capsule, Cellulose, Leucin, and Silica.
E. angustifolia only Products:
APPROVED
Hawaii Pharm Echinacea
Dist. by Hawaii Pharm LLC
1 ml
330 mg angustifolia root powder per ml
TPCs:
Expected Min: 1.7 mg
TPCs: 1.6 mg ✔
Heavy Metals: NAⓘ
Microbial Contamination: NAⓘ
Take about 20-30 drops (0.7-1 ml, one full squeeze of the dropper bulb) to 2-4
oz of juice or water up to 4 times per day.
Liquid from bottle
$0.25/ml
[$1.53]
$29.95/4 fl oz [120 ml] bottle (approx. 120 servings)
Contains No Alcohol. Non-GMO. Gluten Free.
Contains No gluten, alcohol, artificial colors, pesticides, heavy metals.
1 ml
Echinacea (Echinacea Angustifolia) root extract 970 mg. Extraction rate:
330 mg of dry plant material per 1 ml.
Ingredients: Certified Organic Echinacea (Echinacea Angustifolia) dried
root. Origin: USA, more...
Combination Products:
NOT APPROVED
21st Century® Echinacea Complex 250 mg
Mfd. by 21st Century HealthCare, Inc.
2 vegetarian capsules
250 mg (blend of purpurea herb extract and angustifolia root powder)
TPCs:
Expected Min: 5.8 mg to 9.9 mgⓘ
TPCs: 1.3 mg
Heavy Metals: Pass
Microbial Contamination: Pass
Adults take two (2) capsules daily with any meal or as directed by a healthcare
provider.
Large vegetarian capsule
$0.10/2 vegetarian capsules
[$0.78]
$10.46/200 vegetarian capsules
Gluten free. 100% Vegetarian. No added Sugar,
Salt, Yeast, Preservatives, Artificial Flavors or Colors.
2 vegetarian capsules
Echinacea Blend (Echinacea purpurea herb extract & Echinacea
angustifolia root powder) 250 mg.
Other Ingredients: Oat Fiber, Rice Bran, Cellulose, Magnesium Silicate,
Magnesium Stearate, Silicon Dioxide. Contains <2% of: Maltodextrin.
Nutrilite® Immunity Echinacea
Dist. by Amway Corp.
3 tablets
Purpurea root & aerial powder and angustifolia root powder in blend
TPCs:
Expected Min: NAⓘ
TPCs: 25.1 mg
Heavy Metals: Pass
Disintegration: Pass
Microbial Contamination: Pass
Take three tablets daily.
Medium triangular tablet
$0.83/3 tablets
[$0.33]
$33.00/120 tablets
3 tablets
Tricalcium phosphate (in blend), citrus multiflavonoid
complex 100 mg
Halal. Kosher. NSF Contents Certified seal.
3 tablets
Calories 5, Total Carbohydrate 1 g, Sugars <1 g, Triple Guard Echinacea
Blend [Echinacea (purpurea root and aerial parts, angustifolia root), Corn
Starch, Maltodextrin, Tricalcium Phosphate] 506 mg, more...
APPROVED
Top Pick
for a mixture of dried aerial and root portions of E.
purpurea with some E. angustifolia root
Oregon's Wild Harvest Echinacea
Dist. by Oregon's Wild Harvest
2 capsules
330 mg purpurea tops powder
330 mg purpurea root powder
140 mg angustifolia root powder
TPCs:
Expected Min: 5.7 mg
TPCs: 14.7 mg ✔
Heavy Metals: Pass
Microbial Contamination: Pass
Take two capsules twice daily or as directed by your healthcare professional.
Large capsule
$0.38/2 capsules
[$0.26]
$16.95/90 capsules
Certified Organic by Oregon Tilth. Gluten free.
Verified Non-GMO Ingredients. Free from: Dairy, wheat, peanuts, soy, gluten and
corn allergens.
2 capsules
Organic Echinacea tops (Echinacea purpurea) 330 mg, Organic Echinacea
root (Echinacea purpurea) 330 mg, Organic Echinacea root (Echinacea
angustifolia) 140 mg.
Other Ingredients: Non-GMO bovine capsules and nothing else.
APPROVED
Solgar® Echinacea Herb Extract![]()
Mfd. by Solgar, Inc.
1 vegetable capsule
125 mg angustifolia root extract
300 mg purpurea aerial powder
TPCs:
Expected Min: 8 mg
TPCs: 13.2 mg ✔
Heavy Metals: Pass
Microbial Contamination: Pass
As an herbal supplement for adults, take one (1) vegetable capsule up to three
times daily, preferably with meals or as directed by a healthcare practitioner.
Large vegetable capsule
$0.22/vegetable capsule
[$0.17]
$13.34/60 vegetable capsules
Gluten, Wheat & Dairy Free. Suitable For
Vegans. Non-GMO. Free Of: Gluten, Wheat, Dairy, Soy, Yeast, Sugar, Sodium,
Artificial Flavor, Sweetener, Preservatives and Color.
1 vegetable capsule
Standardized Echinacea Extract (Echinacea angustifolia) (root) (echinacosides 5 mg [4%]) 125 mg, Echinacea (Echinacea
purpurea) (aerial) 300 mg.
Other Ingredients: Vegetable Cellulose, Vegetable Magnesium Stearate, more...
Unless otherwise noted, information about the
products listed above is based on the samples purchased by ConsumerLab.com (CL)
for this Product Review. Manufacturers may change ingredients and label
information at any time, so be sure to check labels carefully when evaluating
the products you use or buy. If a product's
ingredients differ from what is listed above, it may not necessarily be of the
same quality as what was tested.
The information contained in this report is
based on the compilation and review of information from product labeling and
analytic testing. CL applies what it believes to be the most appropriate
testing methods and standards. The information in this report does not reflect
the opinion or recommendation of CL, its officers or employees. CL cannot
assure the accuracy of information.
Copyright ConsumerLab.com, LLC, 2021 All
rights reserved. Not to be reproduced, excerpted, or cited in any fashion
without the express written permission of ConsumerLab.com LLC
ConsumerTips™:
What to
Consider When Buying:
Consumers should expect the following information on Echinacea product labels,
and all of this information is required by the FDA (although not all products
comply):
·
The species of Echinacea (i.e., E. purpurea, E.
angustifolia, or E. pallida);
·
The part of the plant used, such as root or the aerial
(above-ground) portions, which include the stem, leaves, and flowers;
·
The form of Echinacea used (e.g., whole herb or root, extract —
which is usually a concentrated form, or tincture);
·
The amount of Echinacea per pill or dose in grams (g) or
milligrams (mg) [1 gram = 1,000 milligrams] (May not be provided for
proprietary blends but preferable if it is.)
Some products may be
standardized to specific substances such as cichoric
acid, alkamides, and polysaccharides. The
concentration of total phenols may also be indicated on the labels of Echinacea
products. The total phenolic content (determined by HPLC) will vary depending
on the type and part of the plant used (see What It Is), but should be about 0.5% to 1%
for quality dried whole herb or root products and proportionally higher for
extracts (based on their listed ratios of Echinacea to total content) but possibly
lower for tinctures, particularly if made from fresh Echinacea (which is about
50% to 75% water).
Many clinical studies suggesting benefit have been conducted with products
extracted from the aerial (i.e., stems, leaves, flowers) portions of E.
purpurea, either as pressed juice extracts or alcohol extracts often dried
into extract powders (900 mg per day) (Linde, Cochrane Database Syst Rev 2006). The
United States Pharmacopeia and WHO additionally recognize the use of the root
from E. purpurea and E. angustifolia.
Because echinacea products come in many forms and concentrations, the dosage
will vary considerably depending on the product. While at first glance it may
seem that products made with a large amount of dried herb powder (sometimes
over 3,000 mg per day) are giving you "more" Echinacea than extracts
(typically several hundred milligrams per day), keep in mind that dry extracts
are generally 4 times the concentration of herb powders depending on the type
of extract. While some herbalists believe that dried herb powders are superior
to extracts because they provide a wider spectrum of plant chemicals, there is
less clinical evidence for dried herb powders of Echinacea than for extracts.
Some Echinacea products may also contain the herb goldenseal (Hydrastis
canadensis). While goldenseal may be useful as an antibacterial agent when
applied directly on topical infections, clinical studies have not shown it to
be useful in treating colds since it is not antiviral and it is specifically
not to be used by pregnant women.
Elderberry is also
an ingredient in some supplements that contain echinacea. Experimental
pharmacological studies and a small number of clinical trials suggest that a
standardized syrup made from elderberry fruits (Sambucus nigra) has an
immune-enhancing effect, which can help reduce infection from colds and flus.
The usual dose for the clinically-tested standardized elderberry syrup ranges
from 1 teaspoon four times daily for children to 2 teaspoons four times daily
for adults for intensive use; regular daily prophylactic maintenance is 1
teaspoon daily for children and 2 teaspoons for daily for adults (ABC Clinical Guide to Elderberry 2004). A
standardized extract of elderberry taken in four doses of 175 mg has recently
shown possible efficacy in preventing flu symptoms in a small pilot trial (Kong 2009). [Note: Although rare, be aware
that a case of acute pancreatitis was reported in
2019 in a 51-year-old man after he took two to three doses of elderberry
extract (Sambucol)].
No meaningful scientific studies have evaluated the combination of Echinacea
with goldenseal or elderberry. However, there have been numerous positive
studies of products that contain fixed combinations of Echinacea plus thuja
(white cedar, Thuja occidentalis) and baptisia (wild indigo, Baptisia
tinctoria), and other combinations of Echinacea preparations with propolis
and vitamin C, or with the extract of the leaf of the immune-modulating
herb Andrographis paniculata.
What to Consider When Using:
In general the total daily dosage of Echinacea is to be divided into two or
three doses taken throughout the day. It is sometimes suggested that liquid
forms be held in the mouth for 10 seconds before swallowing to provide
"local antiviral effects" (Jawad, Evid Based Compl Alt Med
2012).
Some clinical trials, as well as popular use, suggest that Echinacea
preparations are best used as a prophylactic during cold and flu season. They
may also be effective if taken at the first signs of a cold and continued for
one to two weeks. Echinacea (at least, E. purpurea juice) has
not been shown to be helpful in treating colds in children, although one
clinical trial showed a lower incidence of later infections with colds in
children who had consumed a syrup made from the fresh juice of E.
purpurea (Taylor, 2003).
Concerns and Cautions:
Echinacea
taken by itself appears to be relatively safe. The herb has been used safely in
trials lasting up to 12 weeks but is not recommended for long-term use.
Reported side effects are minor and include non-specific
gastrointestinal symptoms and increased urination.
Some people may be allergic to Echinacea, and it has been
noted that people allergic to sunflowers and other flowers in the daisy family
run a higher risk of having an allergic reaction. One large study found that
children given Echinacea were somewhat more likely to develop a rash than
those given placebo, but otherwise no harmful effects were seen.
Because Echinacea is thought to work by stimulating the immune system, this
herb is generally not recommended for people with autoimmune diseases
or those taking immune-suppressant drugs.
Two cases of acute hepatitis (liver inflammation) (one in
Turkey and one in Greece) have been reported with the use of Echinacea root
tablets (600 mg - 1,500 mg per day -- species not given) which resolved within
one to three months of stopping supplementation (Kocaman,
Eur J Intern Med 2008; Gabranis, J Postgrad Med
2015).
Preliminary research suggests Echinacea purpurea root could
potentially increase the effects of caffeine, as it has been shown
to increase blood concentrations of caffeine by as much as 30% due to an
inhibitory effect on the CYP1A2 metabolic enzyme (Gorski, Clin Pharmacol
Ther 2004).
Echinacea may potentially affect the metabolism of drugs
metabolized by the CYP3A4 enzyme, although it has been noted
that, if used per label recommendations, Echinacea supplements are not likely
to have a dramatic effect (Gurley, Planta Med 2012). Drug metabolized by
CYP3A4 include lovastatin (Mevacor), atorvastatin
(Lipitor), simvastatin (Zocor), clarithromycin (Biaxin), cyclosporine (Neoral, Sandimmune), diltiazem
(Cardizem), estrogens and triazolam (Halcion) (Budzinski, Phytomedicine 2000).
It may also affect the metabolism of warfarin (Coumadin), although clinically
significant changes in INR have not been reported (Coumadin Prescribing Information 2019; Abdul, Br J Clin Pharmacol 2010).
There is little information on the safety of Echinacea for
very young children or pregnant or nursing women. One review article indicates
that using Echinacea during the first trimester of pregnancy may be safe,
though it is not recommended during lactation until further studies are done (Perri, Can J Clin Pharmacol
2006). Women who may become pregnant while using Echinacea should
keep this in mind.
Information on this site
is provided for informational purposes only. It is not an endorsement of any
product nor is it meant to substitute for the advice provided by physicians or
other healthcare professionals. The information contained herein should not be
used for diagnosing or treating a health problem or disease. Consumers should
inform their healthcare providers of the dietary supplements they take.
Latest Clinical Research Updates for Echinacea Supplements
7/17/2021
Can echinacea reduce
symptoms of mild to moderate anxiety? See what a recent study found in
the What It Does section of our Echinacea
Supplements Review. Also see our Top Picks among echinacea supplements.
Also see our answer to the question: What are the
best supplements for depression and anxiety?
10/21/2012
Although the evidence
behind echinacea has been mixed, a new study -- the largest to date -- found
that taking a particular type of echinacea supplement throughout cold season
reduced cold "events" (catching colds and days spent with colds) by 26%.
See the update to the Echinacea Supplements Review for details
about this particular supplement, including the special way it is taken. More >>
Does Echinacea Help Treat a Cold?
1/02/2011
Very little, it seems. A
new large, well-controlled study of a commercially sold echinacea supplement
failed to show a substantial benefit when given shortly after the first sign of
a cold. Evidence tends to be better (although not robust) for echinacea used to
prevent a cold before it begins, as opposed to once symptoms
start. Find out more (including the product that was tested) in the Echinacea
Supplements Review. More >>
Related CL Answers (10)











