CBD & Hemp Extract Supplements, Lotions, and Balms
Review
Find the Best CBD at the
Lowest Price! Learn How Much CBD (and THC) Is Really in Products and Which Are
Our Top Picks.
Medically reviewed and
edited by Tod Cooperman, M.D. 
Last Updated![]() : 09/17/2021 | Initially Posted:
09/05/2020Latest Update: Help for Arthritic Hands?
: 09/17/2021 | Initially Posted:
09/05/2020Latest Update: Help for Arthritic Hands?


Table of Contents
Summary
·
What is CBD? CBD (cannabidiol) is a compound derived from hemp and
marketed as a supplement despite the U.S. FDA's position that CBD is not a
dietary supplement.
·
Does CBD work? CBD taken orally has been shown to
reduce the frequency of certain types of seizures, and preliminary evidence
suggests it may also help with anxiety, schizophrenia, and other
conditions. However, most of these effects have involved large
doses of CBD — hundreds of milligrams per day, which is more than in many
marketed CBD supplements and products. CBD applied to the skin (such as CBD
creams, gels, and lotions) may modestly reduce some forms of pain (See What It Is and What It Does).
·
What did CL's tests of CBD products find? ConsumerLab found
significant amounts of CBD in all of the products (ranging from 2.5 mg to 51.3
mg per serving) but the cost to obtain an equal amount of CBD from each
product ranged more than 10-fold, from just 24 cents to $2.67 per 10 mg.
Interestingly, there were many good, lower cost products available on the
market than ConsumerLab found in its last Review in 2018. Levels of THC
(tetrahydrocannabinol, a psychoactive compound) also tended to be much lower,
with THC not detectable in most products. (See What CL Found and use the Results table to compare the amounts of
CBD, THC, and other cannabinoids in products).
·
Best CBD Oil? Based on quality and value, ConsumerLab
selected several Top Picks including
an overall Top Pick as well as high, medium, and low-dose picks
for oral use, a Top Pick among topical lotions and
balms, and a Top Pick for
Pets based on top quality and value.
·
How to choose a CBD product? If you seek CBD, look for products
that list the amount of CBD or cannabidiol per serving (and
don't confuse that with the amount per entire bottle). If a product lists only
"cannabinoids" it may contain some CBD but you won't know how much.
Products may still have significant amounts of CBD if they list
"hemp extract" as an ingredient, but don't expect much
CBD if "hemp oil" is the only ingredient. Hemp extracts are
more likely to contain a range of cannabinoids in addition to CBD (although
it's not clear if this provides added benefit) and this is what is meant by the
term "full-spectrum" on labels — but full-spectrum products may or
may not contain THC. If you want to avoid THC, look for products that claim to
be "THC-free." (See ConsumerTips™)
·
How much CBD should I take and when? Most of the
research with CBD has involved high doses (several hundred milligrams daily).
However, many CBD products on the market are lower dose and it is not clear if
this dosing is effective. Nevertheless, to greatly increase CBD absorption,
take it with or shortly after a fatty meal. (See ConsumerTips™: Dosage)
·
CBD safety, side effects and drug interactions: High-dose CBD can
cause a range of side effects (particularly gastrointestinal) and affect
certain medications. For details, see Concerns and Cautions.
What It Is:
Cannabidiol
(CBD) and its precursor compound CBDa are dominant "cannabinoid"
compounds found in hemp and cannabis (a hemp plant also known as marijuana).
When CBDa gets converted to CBD, most of it becomes CBD; consequently, 87.7%
CBDa in a product can be "counted" as CBD. Unlike tetrahydrocannabinol
(THC), which is another cannabinoid compound, CBD is not believed to be a
psychoactive compound affecting perception and behavior.
What It Does:
NOTE: The effects described below are primarily based on
daily doses of hundreds of milligrams of CBD. Many CBD products on the market
contain much lower amounts (providing tens of milligrams or less per day), and
it is not known if these low doses are as effective as higher doses.
Epilepsy and seizure disorders
Much of the research with CBD has focused on the reduction of certain types of
seizures. A placebo-controlled clinical trial found a high daily dose of CBD
(20 mg per kg of body weight, i.e., hundreds of milligrams) to reduce the
frequency of convulsions in a rare form of epilepsy known
as Dravet syndrome in children and young adults, although it
was also associated with a higher rate of adverse effects including diarrhea,
vomiting, fatigue, pyrexia, somnolence, and abnormal results on liver-function
tests (Devinsky, New Eng J Med 2017).
Similarly, the same high daily dose reduced the number of drop seizures among
people with treatment-resistant Lennox-Gastaut syndrome in a
3-month study. Seizures per month decreased 44% with CBD compared to 22% with
placebo; however, again, those taking CBD also had a higher rate of adverse
effects including diarrhea, somnolence, fever, decreased appetite, and vomiting
(Thiele, Lancet 2018).
Both studies noted above were funded by GW Pharmaceuticals,
the maker of the prescription oral solution of cannabidiol Epidiolex.
It should be noted that after reviewing these studies, the United Kingdom's
National Institute for Health and Care Excellence rejected Epidiolex as
a drug for treating these seizures in its draft guidance. The agency raised
concerns about the trials, particularly their short duration, because other
antileptic drugs are known to lose their effectiveness over time (Dijk, BMJ 2019).
A study in Nashville among 108 children with refractory epilepsy who had been
treated with commercially marketed CBD preparations found that CBD could be as
effective as the anticonvulsant drug clobazam when used as add-on therapy. A
reduction in seizure activity of at least 50% occurred in 33%, 38%, and 44% of
those who received, respectively, CBD, clobazam, and CBD+clobazam. Relative to
clobazam, CBD seemed to increase alertness and increase verbal interactions.
Sedation, which was common among those taking clobazam, did not occur among
those taking only CBD. The average daily dose was about 3 mg of CBD per
kilogram of body weight: e.g., 100 mg per day for a 73 lb child. (Porcari, Epilepsy & Behav 2018).
Parkinson's disease and movement disorders
CBD does not appear to improve motor symptoms of Parkinson's disease, such as
tremor and difficulty initiating movement, or involuntary movements
(dyskinesia) associated with the use of the anti-Parkinson's drug levodopa.
However, CBD may be helpful for non-motor symptoms of
Parkinson's disease, such as psychosis, mood and sleep disturbances —
although more research is needed (Crippa, Eur Arch Psychiatry Clin Neurosci 2019).
For example, there have been some preliminary reports of CBD oil (150 mg to 400
mg) improving symptoms of psychosis, with no worsening of motor function, in
individuals with Parkinson's disease and psychosis who were taking levodopa (Zuardi, J Psychopharmacol 2009). In three
older men with Parkinson's disease and REM sleep behavior disorder
(RBD) (characterized by intense dreams and behavior such as laughing,
yelling, kicking and punching during sleep) who experienced disruptive sleep
episodes between two and seven times per week, none experienced these symptoms
during a six-week period of daily dosing with 75 mg of CBD. A fourth man, who
took 300 mg of CBD daily for the same time period had a reduction in episodes
from two to four times per week to once per week (Chagas, J Clin Pharm Ther 2014). A small,
double-blind, placebo-controlled study in Brazil found no improvement in
motor function in men and women with mild to moderate Parkinson's disease
without other psychiatric conditions (average duration of disease 8 years) who
took CBD oil (75 mg or 300 mg) daily in addition to their regularly prescribed
anti-Parkinson medication for six weeks -- but those who took the 300 mg dose
had significant improvements in Parkinson's Disease Questionnaire-39 total
scores, and measures of quality of life such as activities of daily living,
"stigma" and emotional well-being. No adverse effects were associated
with the use of the CBD oil (Chagas, J Psychopharmacol 2014).
A preliminary trial found modest, dose-related improvements in symptoms
of dystonia (involuntary muscle contractions) in five
individuals taking between 100 mg and 500 mg per day of cannabidiol; however,
at the highest dosages (300 mg to 500 mg) there was a worsening of
tremor and the ability to initiate movement in two of the individuals
who also had Parkinson's disease (Consroe, Int J Neurosci 1986).
Although the cause of essential tremor (ET) is
still unknown, laboratory research has shown that cannabinoid receptor type 1
(CB1) antagonists can reduce tremors in animal models of ET (Abbassian, Br J Pharmacol 2016). Because
cannabidiol acts as a CB1 antagonist and has shown some, although mixed,
evidence of benefit for other types of tremors, there has been interest in
using it to reduce symptoms of ET. However, a small study that included 19
people with ET, all of whom were undergoing drug treatment, found that a single
dose of 300 mg of cannabidiol did not reduce the severity of upper limb tremors
compared to placebo (Santos de Alencar, Parkinsonism
Relat Disord 2021). It is unclear if long-term treatment with
cannabidiol would be beneficial.
Schizophrenia and psychosis
Results in people with schizophrenia have
been mixed. A study of high-dose CBD (500 mg taken morning and evening) among
adults with schizophrenia found that adding CBD rather than placebo to existing
treatments for six weeks reduced psychotic symptoms and caused a trend,
although not statistically significant, toward improved performance on
cognitive tasks. In this study, CBD was well tolerated with no increase in
adverse effects (McGuire, Am J Psy 2017). Another 6-week study, however, found that 300 mg of CBD taken
twice daily did not improve cognitive or psychotic symptoms in adults with
schizophrenia on stable doses of medication; in fact, only those taking a
placebo experienced a (modest) improvement in symptoms. Twenty percent of
CBD-treated patients experienced sedation (mostly mild) compared to 5% of those
on placebo (Boggs, Psychopharm 2018).
CBD seems to "partially normalize
alterations" in areas of the brain that are implicated in psychosis (i.e.,
severely impaired thoughts and/or emotions). This was shown in a small,
placebo-controlled study that measured activation of areas of the brain (based
on blood flow measured with MRI imaging) during a verbal learning task. Brain
activity in people at high risk of psychosis given a single dose of 600 mg of
CBD more closely resembled that of people not at risk of psychosis than of
people at risk of psychosis who were given a placebo. Interestingly, THC in
marijuana can have the opposite effect and has been associated with the
development and relapse of psychosis (Bhattacharyya, JAMA Psych 2018).
A possible mechanism for an antipsychotic effect of CBD is that, at a dose of
600-800 mg per day, it seems to elevate natural levels of the endocannabinoid
anandamide by moderately inhibiting its degradation (Rohleder, Front Pharmacol 2016).
Anxiety, PTSD, and sleep
CBD may reduce anxiety and symptoms of PTSD (post-traumatic stress
syndrome). It may also improve sleep in people with such disorders and those
with insomnia, but does not appear to be helpful as a sleep aid in healthy
individuals.
Studies in animals as well as several small studies in people suggest CBD may
help reduce anxiety. For example, a small placebo-controlled study
in young healthy men found that a single 400 mg dose of CBD taken as a capsule
reduced self-reported anxiety (but also increased feelings of mental sedation)
one hour after ingestion (Crippa, Neuropsychopharmacology
2004). Studies have also found CBD to reduce anxiety with social
speaking when given 90 minutes before speaking at a dose of 300 mg, while a
lower dose (150 mg) was not effective and there were mixed results with a
higher dose (600 mg) (Bergamaschi, Neuropsychopharm 2011), Zuardi, J Psychopharmacol 1993, Linares, Braz J Psychiatr 2018). A study among Japanese teenagers with social anxiety found
that 300 mg of CBD (which contained no THC and was in a base of MCT oil) daily for four
weeks led to a modest decrease in symptoms while there was no significant
change in symptoms among those given placebo (olive oil) (Masataka, Front Psychol 2019).
Case reports from an outpatient mental health clinic in
Colorado suggested that even lower doses of CBD might be helpful with anxiety
and related sleep disorders. (Bear in mind that case reports are not
controlled studies, so these benefits remain to be proven.) In a group of 72
men and women treated with CBD daily for two months, anxiety scores decreased
within the first month in 79.2% of patients and remained decreased during the
study duration. Sleep scores improved within the first month in 66.7% but
fluctuated over time. It's important to note that 15.3% reported a worsening of
anxiety and 25% reported a worsening of sleep, and similar percentages were
reported in the second month of supplementation. Most of the participants took
one capsule daily containing 25 mg of CBD oil (provided by CV Sciences Inc.,
makers of PlusCBD Oil, although it was not a sponsor of the study),
and continued taking their regularly prescribed medications. For anxiety, CBD
was taken after breakfast, while, for sleep, it was taken after dinner. CBD was
generally well-tolerated, although two patients discontinued treatment within
the first week because of fatigue and three noted mild sedation that appeared
to abate after a few weeks of treatment. CBD was discontinued for one patient
with a developmental disorder in whom treatment appeared to cause disinhibition
in the form of inappropriate sexual behavior (Shannon, Perm J 2019).
Another series of case reports from the clinic in Colorado
suggested that CBD may help relieve symptoms of post-traumatic stress
disorder (PTSD). In a group of 11 adults with diagnosed PTSD, overall
symptoms decreased by an average of 28% over a 2-month period of CBD
supplementation. The average daily CBD dose was 33.4 mg at the start,
increasing to 44.6 mg by the end of the study. The participants took CBD as 25
mg capsules and/or an oral spray (1.5 mg of CBD per spray) (Elms, J Altern Complement Med 2019).
Separately, the same clinic reported that CBD appeared to be helpful to a
10-year old girl with anxiety and sleep disorder due to PTSD caused by sexual
abuse. She took 25 mg of CBD at bedtime and 6 mg to 12 mg of CBD from a
sublingual spray as needed throughout the day for 5 months. Her anxiety and
sleep improved to the extent that they were no longer classified as disorders (Shannon, Perm J 2016). Although no side
effects were observed, it is important to note that there is concern that
cannabinoids may affect brain development in children (see Concerns and Cautions).
Preliminary research suggested that CBD may affect the sleep-wake
cycle, although this may depend on the dose and the condition for which it is
taken. Low-dose CBD (15 mg) may have a stimulating effect,
while moderate and higher doses can be sedating and may improve sleep in people
with anxiety (as in the case report above) and in those with certain sleep
disorders (Babson, Curr Psychiatry Rep 2017).
For example, a small study among 15 men and women with a history of insomnia found
that 160 mg of CBD taken as a capsule 30 minutes before bedtime for one week
significantly increased self-reported duration of sleep compared to placebo.
Ten participants reported sleeping more than 7 hours after taking this dose of
CBD, but when the same participants took a placebo, only six reported getting
more than 7 hours of sleep. However, there was no decrease in the amount of
time it took to fall asleep. Lower doses of CBD (40 mg and 80 mg) did not
increase sleep time or reduce the amount of time it took to fall asleep (Carlini, J Clin Pharmacol 1981). None of the
participants reported increased difficulty in waking or feeling sleepy upon
awakening, compared to placebo.
On the other hand, an even greater dose of CBD -- 300 mg taken 30 minutes
before bedtime, had no effect on the time it took healthy men
to fall asleep. It also had no effect on the amount of time spent in each stage
of sleep (such as REM sleep) or the amount of time participants stayed asleep
(as measured by polysomnography), and it did not affect self-reported sleep
quality, compared to placebo. CBD was not found to impair cognitive function
when evaluated the following morning (Linares, Front Pharmacol 2018).
A study among 65 healthy, overweight men and women in the
U.S. found that 15 mg of CBD from 60 mg of hemp oil extract (PlusCBD Extra
Strength Hemp Extract, CV Sciences) taken as a softgel once daily with
breakfast for six weeks did not improve sleep quality, mood, or ability
to cope with stress compared to placebo. CBD supplementation modestly
increased blood levels of "good" HDL cholesterol, but
there were no changes in total or "bad" LDL cholesterol, body weight,
blood pressure, or bodyweight compared to placebo. No increases in liver
enzymes or serious adverse events were reported (Lopez, J Diet Suppl 2020). The hemp extract
used in the study closely resembles that in Plus CBD Oil Hemp Softgels (60
mg hemp oil) tested and approved in this review.
A study in Brazil gave 118 physicians, nurses and
physical therapists caring for patients hospitalized with COVID-19 high-dose
oral CBD drops (150 mg taken twice daily) for 28 days in addition to standard
care (motivational and instructional videos on physical exercise and weekly
consultations with psychiatrists) or standard care alone. Compared to those
given only standard care, the CBD group had a slightly greater reduction in
symptoms of emotional exhaustion (by 4.14 points on a 54 point
scale) and anxiety (a 30% decrease in symptoms vs only 2%).
Although symptoms of burnout decreased among those who took
CBD compared to baseline, the change was not statistically significant. Those
who took CBD were more likely to report increases appetite (23,7% vs 8.5%).
Four participants who took CBD experienced an increase in liver enzymes
(including one severe case) and one experienced a severe adverse skin reaction,
while there were no adverse reactions in the control group. The extract used in
the study was 99.6% CBD, dissolved in medium-chain triglyceride oil (by PurMed
Global, which funded the study. Unfortunately, a placebo was not given to the
control group, which would have made the results more meaningful.) (Crippa, JAMA Netw Open 2021).
Substance use disorder
High-dose CBD (400 mg or 800 mg of the prescription CBD oral solution
Epidiolex) was shown to reduce drug cravings in a placebo-controlled study
among 42 men and women with heroin use disorder, most of whom
had been abstinent from heroin for less than one month. CBD was taken once
daily for three days. CBD reduced cue-induced drug craving and anxiety and also
reduced physiological measures of cue-induced craving such as increased heart
rate compared to placebo one to two hours after the first dose of CBD as well
as on the seventh day. Both doses of CBD equally reduced craving. There were no
significant effects on cognition and no serious adverse events; mild side
effects such as diarrhea, headache and fatigue were reported in both those
taking CBD and placebo (Hurd, Am J Psychiatry 2019).
CBD may have helped a 17-year old male in Vienna,
Austria with multiple substance use disorder (cannabis, MDMA, cocaine,
ecstasy), severe depression, social phobia and narcissistic personality
disorder. After unsuccessful treatment with the antidepressant sertraline for 6
months, he was started on a dose of 100 mg CBD (50 mg in the morning and
evening), which, after 3 weeks, was gradually increased to 600 mg (300 mg twice
daily), for a total treatment period of 8 weeks during which he also received,
in a day clinic setting, group and individual psychotherapy, social cognition
training, occupational therapy and physiotherapy, and his parents had weekly
meetings with the treating psychiatrist. He reported no side effects from the
CBD. He improved regarding depressive as well as anxiety symptoms and quit
abusing illegal drugs without showing withdrawal symptoms (Laczkovics, Neuropsychiatr 2020).
The use of CBD to treat cannabis use
disorder appears to require a fairly high dose. A 4-week placebo-controlled
clinical trial that included 82 people (average age 26) with cannabis use
disorder showed that taking 100 mg of CBD twice daily was not effective,
but 200 mg twice daily provided a modest benefit, which was marginally better
than a higher dose of 400 mg twice daily. At 200 mg twice daily, cannabis users
abstained from use about half a day more per week than those given a placebo.
The reduction in cannabis use was confirmed by a decrease in urinary excretion
of a THC metabolite (Freeman, Lancet Psychiatry 2020).
Glaucoma
Although there is some preliminary evidence that THC and, possibly other
cannabinoids could potentially help to reduce interocular (eye) pressure in
people with glaucoma, one study found that, four hours after
ingestion, a single, sublingual dose of CBD (which also contained about 1 mg of
THC) had no effect on interocular pressure, while a 40 mg dose of CBD
(containing about 2 mg of THC) temporarily increased interocular
pressure (Tomida, J Glaucoma 2006; Health Canada 2013).
There do not appear to be longer-term studies, or studies investigating the
effects of cannabidiol alone for glaucoma.
Pain
Preliminary research suggested a role for CBD in pain management but, in
most cases, studies used combinations of THC with CBD rather than CBD alone or
with minimal CBD. When given without THC, there is currently no evidence that
CBD reduces low back pain, arthritic pain, or other pain conditions.
A study in Australia among 100 men and women (median age 47)
treated in a hospital emergency room for acute low back pain (not
due to trauma) found that taking a single, high dose of CBD (400 mg in MCT oil)
in addition to standard treatment (1,000 mg paracetamol (Tylenol) + 400 mg
ibuprofen) did not reduce pain any better than giving a
placebo (MCT oil alone) with the same standard treatment. In addition, the CBD
did not reduce the need for rescue medication (oxycodone) or shorten the length
of hospital stay compared to placebo. The occurrence of side effects such as
sedation, light headedness, diarrhea and nausea were similar in both groups (Bebee, Med J Aust 2021).
A study showed promise with CBD for people (ages 39 - 70)
with chronic pain who had been on opioids for at least one
year; however, the study lacked a placebo control, so it is not possible to
know if the effects were due to CBD. Study participants were given softgels of
hemp extract (15.7 mg CBD and 0.5 mg THC per softgel, from Ananda Professional,
which funded the study. For eight weeks, most participants added two of the CBD
softgels (a total of 31.4 mg of CBD) to their daily medication regimens. Among
94 patients who completed the study, 94% reported quality of life improvements
and 53% reduced their use of opioids (three of whom completely eliminated
opioids). CBD appeared to modestly reduce pain intensity and improve sleep
quality. Although there were trends toward improvements in pain-related
disability and overall health, these were not statistically significant. No
significant adverse events were reported, but a small number of participants
experienced nausea, heart burn, dry mouth, and/or nighttime anxiety, and two
people did not complete the study due to drowsiness (Capano, Postgrad Med 2019).
One study found that an oral spray containing THC and CBD reduced pain in
people with rheumatoid arthritis (Blake, Rheumatology (Oxford) 2006); however, a
review of four short term clinical studies (including this one) investigating
the effects of cannabinoids for the treatment of rheumatic diseases,
including fibromyalgia syndrome, back pain, osteoarthritis and
rheumatoid arthritis, concluded that there is "currently insufficient
evidence to recommend cannabinoid treatments for management of rheumatic
diseases pending further study." (Fitzcharles, Schmerz 2016). Furthermore, a three-month study in Denmark among 129
men and women with moderate chronic pain due to hand osteoarthritis or
psoriatic arthritis found that a daily dose of CBD that started at 10
mg for two weeks and then increased to 20 or, eventually, 30 mg (taken as a 10
mg tablet with meals, up to three times daily), did not decrease pain or
improve self-reported sleep quality, depression or anxiety compared to placebo
(Vela, Pain 2021).
A review of several experimental pain studies (using heat or
pressure) on healthy people found that cannabis (marijuana) and cannabinoids
may not reduce the intensity of pain, but may make pain feel less
unpleasant and more tolerable. Although none of the studies tested CBD
exclusively, the products containing CBD (in addition to THC), such as cannabis
extracts, were shown to be more effective than those containing exclusively THC
or THC analogues (De Vita, JAMA Psych 2018). However,
a subsequent experimental pain study found that CBD alone did not reduce pain
threshold, tolerance or severity compared to placebo, and suggested that a
reduction in pain "unpleasantness" may largely be due to the placebo
effect — the expectation that CBD will reduce pain. In the
study, participants who were given 50 mg of CBD orally as a liquid (99.46% CBD
in coconut oil with no detectable THC) but were told they were taking a placebo,
30 minutes before a pain-inducing heating element was applied to the forearm,
reported a similar reduction in "unpleasantness" as those who were
given a placebo (coconut oil) but told they were given CBD (De Vita, Exp Clin Psychopharmacol 2021).
A few studies suggest that a combination of THC and CBD may be helpful
for cancer-related pain; however, there do not appear to be studies
on the effects of CBD alone for cancer-related pain (Blake, Ann Palliat Med 2017).
Cancer
Preliminary evidence suggests that CBD may have anti-cancer properties and
enhance the immune response to cancer. Although clinical studies have not been
conducted, a case was reported in the UK of a man in his early 80s with lung
cancer (biopsied as adenocarcinoma) who, after declining traditional therapies,
had a dramatic reduction in tumor size after self-administering low-dose CBD
twice daily (starting at just 1.32 mg of CBD twice daily for one week, and then
6 mg of CBD twice daily for another three weeks — each dose consisted of 9
drops (0.3 mL) of "MyCBD" oil labeled as 2% CBD. He took this daily
dose again for a week after learning of the tumor reduction but stopped as he
did not like the taste and it caused him slight nausea. A scan two months later
showed the tumor remained stable in size. Further research is needed on CBD's
effect on cancers (Sule-Suso, Sage Open Medical Case
Reports, 2019). (For more information about cannabinoids and cancer
treatment, see the National Cancer Institute's webpage about this
topic.)
Colds, flu, viral infections
CBD has been promoted to fight colds, the flu, and even viral infection with
SARS-CoV-2 (which causes COVID-19), but there is
currently no direct clinical evidence to support these uses. In fact, when high
doses of CBD (hundreds of milligrams per day) were given to children and young
adults to determine CBD's anti-seizure effects (see Devinsky study, above), the number of upper
respiratory infections reported was slightly higher among those who took CBD
compared to placebo (11% vs. 8%, respectively).
On the other hand, laboratory research has found that CBD
inhibits the replication of SARS-CoV-2 in animal cells and human lung cells,
and interestingly, THC and other cannabinoids diminished CBD's
antiviral effects. The same researchers who conducted these laboratory studies
looked at COVID infection rates in people taking high-dose CBD. Their analysis
of 85 patients tested for SARS-CoV-2 at the University of Chicago Medical
Center who were also taking high-dose, prescription CBD (Epidiolex) before
getting tested (at any point or up to 2 years before COVID testing) found that
only 1.2% tested positive for COVID-19, compared to 12.2% who tested positive
among a matched control group who were not taking CBD (Nguyen, bioRxiv 2021 -- preprint).
However, only randomized, placebo clinical trials can determine if CBD reduces
the risk of infection.
At the same time, animal experiments suggest CBD may to dampen immune
system response to infection, by decreasing inflammatory cytokines and the
production of immune system cells (Nichols, Cannabis Cannabinoid Res 2020). For
example, CBD has been shown to decrease the inflammatory response to infections
such as pneumococcal meningitis (Barichello, Eur J Pharmacol 2012).
This dampening of the immune response could potentially have benefit in
treating autoimmune diseases, as demonstrated in an experimental model of
multiple sclerosis in mice (Kozela, Br J Pharmacol 2011). It could be
hypothesized that this could also dampen the devastating "cytokine
storm" that occurs in severe cases of COVID-19, but it far too early to
know if this would be the case or whether it might make the infection worse.
Topical CBD
Creams, gels and lotions containing CBD are often promoted to treat
pain, such as muscle or joint pain. CBD appears to be better absorbed through
the skin than THC (Huestis, Chem Biodivers 2007) and there is
some evidence that, in animals, creams and gels containing CBD may help reduce
inflammation in conditions such as arthritis and multiple sclerosis (Hammell, Eur J Pain 2016; Giacoppo, Daru 2015). However,
to date, clinical studies have not provided convincing evidence of a benefit
with topical CBD. At best, topical CBD may provide a modest benefit
to people suffering from intense forms of pain, with little demonstrated
benefit in cases of less intense pain.
In a study of 13 young adults, CBD cream applied to the front thigh area after
leg exercises (a series of squats) did not decrease delayed
onset muscle soreness (DOMS) in the 48 hours following exercise
compared to placebo (petroleum jelly). The cream contained 200 mg of CBD in
each 1-oz. container (6.7 mg CBD/mL), which was shared among all of the
participants, although the specific amount of cream or CBD was not specified (Garcia, Int J Exc Sci 2019 - Abstract
plus correspondence with ConsumerLab). In the other study, a gel containing
10.5 mg or 21 mg of synthetic CBD was applied daily to the knees of people
with knee osteoarthritis. Although both groups experienced
reductions in "worst knee pain" scores, these reductions were not
statistically significant compared to the reduction in pain scores for placebo
group that used gel without CBD. Dryness at the site of application and
headache were more frequent among those who used the CBD. The study was
conducted by a company (Zynerba) seeking to develop the synthetic CBD (ZYN002)
as a drug (Hunter, Osteoarthritis Cartilage
2018).
A preliminary study suggests that CBD cream may reduce certain
symptoms of peripheral neuropathy, a condition characterized by
nerve pain, burning, "pins and needles" sensations and weakness,
typically in the hands and feet. In the study, 29 men and women (average age
68) with peripheral neuropathy of the lower extremities (feet and lower legs)
due to type II diabetes, chemotherapy treatment, or other causes received one 3
fl. oz container of CBD cream blended with emu oil to enhance absorption (Theramu
Relieve CBD compound cream -- 250 mg of CBD per container, 2.8 mg
CBD/mL) or placebo cream (similar cream with emu oil but no CBD) and applied
the cream to symptomatic areas up to four times daily for one month.
Participants who used the CBD cream had modest, but statistically significant
reductions in self-reported intense pain, sharp pain, cold and itchy
sensations, but no reduction in hot, dull, sensitive, unpleasant, deep and
surface pain, compared to placebo. No adverse events were reported. Theramu supplied
the cream but did not fund the study (Xu, Curr Pharm Biotechnol 2019).
A clinical trial that suggested some benefit with topical
CBD studied 60 young adults with temporomandibular disorder (jaw muscle
pain from teeth grinding) in Poland. Participants rubbed a
"pea-size" amount of ointment over their jaw muscles twice a day. For
half the group, CBD Oil (Charlotte's Web Hemp Extract Oil in Olive Oil --
67 mg CBD/mL) was added to the ointment (comprising 20% of the total ointment,
making it 1.46% CBD, or about 7 mg of CBD per 0.5 gram application). After 14
days, jaw muscle activity at rest had decreased by about 12% in the CBD group
versus only about 2% in control group, although this was not a statistically
significant difference. Perhaps more importantly, the CBD group reported a 70%
reduction in pain intensity versus only a 10% reduction for the control group,
although the statistical significance of this difference was not determined (Nitecka-Buchta, J Clin Med 2019).
CBD for Pets:
Preliminary evidence suggests that short-term CBD supplementation may be of
modest benefit to dogs with osteoarthritis. There is some concern, however,
with the effects of CBD on liver enzymes in dogs and cats, as discussed below.
A study at Cornell University found that giving older dogs with
osteoarthritis CBD, compared to placebo, for one month modestly
reduced pain and increased activity levels (rising to standing, walking,
running, and climbing) as reported by the dogs' owners, and reduced joint pain
upon touch when examined by a veterinarian. However, there were no improvements
in lameness or weight-bearing (i.e. reluctance to rise, favoring the affected
leg when walking, or limping) as assessed by a veterinarian. The dose was 2 mg
of an equal mix of CBD and CBDa per kg of bodyweight given twice daily (e.g.,
for a 20 lb dog: 18 mg of CBD/CBDa in the morning and again at night), and use
of other regular supplements (e.g., glucosamine, fish oil, etc.) and NSAIDS was
allowed. No side effects were reported with CBD but there was an increase in
levels of the liver enzyme alkaline phosphatase, and the researchers
recommended monitoring liver enzymes in dogs receiving CBD until long-term
safety studies are conducted. The CBD (a hemp extract in olive oil) was
provided by ElleVet Sciences, which funded the study (Gamble, Front Vet Sci 2018).
The same ingredient used in the above study was the subject
of preliminary, 12-week, safety studies in young, healthy dogs and cats sponsored
by the manufacturer. The dogs were given CBD as a softchew (ElleVet Mobility
Chews by ElleVet Sciences) at a dose of 2 mg of CBD/CBDa per kg of
bodyweight twice daily (e.g., 18 mg of CBD/CBDa in the morning and again at
night for a 9 kg, or 20 lb, dog) and this did not significantly increase liver
enzymes or cause adverse effects other than a low incidence of loose stool
(3.3% of the time) and vomiting (<1% of the time). Food consumption and
bodyweight remained consistent, and there were no abnormalities or changes in
behavior noted. The cats were given the same ingredient as CBD-infused fish oil
in capsules also at 2 mg CBD/CBDa per kg of bodyweight (e.g., for a 4 kg (9 lb)
cat, 8 mg CBD/CBDa in the morning and again at night). One of the eight cats
studied had a significant increase in a liver enzyme (alanine amino
transferase, or ALT). The most common adverse effects were likely due to the
capsules (some of which broke when being given to the cats) and included heavy
salvation/drooling, excessive licking, gagging, and head shaking. There were no
changes in food consumption, bodyweight or behavior. Interestingly, in cats,
the bioavailability of CBD/CBDa was only about one-fifth of that in dogs, which
would suggest either much lower absorption or faster elimination. The
researchers concluded that "...CBD-rich hemp nutraceuticals appear to
be safe in healthy adult dogs, while more work in cats is needed to fully
understand utility and absorption." (Deabold, Animals (Basel) 2019).
A placebo-controlled study in large dogs with
osteoarthritis found that 20 mg of CBD in a liposomal formulation or 50 mg of
CBD alone taken daily for 4 weeks significantly decreased pain and increased
mobility with no adverse effects on metabolism, hematocrit or other blood
chemistry. The beneficial effect remained for at least 15 days after cessation
of therapy. A 20-mg dose of CBD not in liposomal form was not effective.
Additional laboratory research by the investigators indicated that CBD
exhibited anti-inflammatory effects, reducing the production of tumor necrosis
factor-alpha (TNF-a) and increasing the production of anti-inflammatory
interleukin-10 (IL-10) (Verrico, Pain 2020).
Legality, Quality
Concerns, and Tests Performed:
Legality
Although CBD is not psychoactive, it is not permitted to be
sold as an ingredient in dietary supplements in the U.S. as it is a
FDA-approved drug and, prior to that approval, the FDA considered CBD an
investigational new drug. (Note: If an ingredient is marketed as a supplement
prior to the FDA authorizing its investigation as a drug, it may continue to be
marketed as a supplement, but this was not the case with CBD, according to the
FDA).
The FDA reiterated its position in
November 2019 that CBD is not a dietary supplement and cannot be added to a
food, adding that it " ...has seen only limited data about CBD safety and
these data point to real risks" and that "Some CBD products are being
marketed with unproven medical claims and are of unknown quality." CBD
products are not permitted on flights unless they contain no more than 0.3
percent THC on a dry weight basis or have been approved as drugs by the FDA
(e.g., Epidiolex), according to the Transportation Security
Administration (TSA). TSA security officers do not search for
CBD or marijuana, but, if any illegal drugs is observed during security
screening, TSA will refer the matter to a law enforcement officer.
On December 12, 2018, the U.S. Farm Bill passed,
permitting farmers to legally grow hemp like any other crop (although this does
not extend to marijuana - the strain of hemp higher in THC). While this will
probably not change the FDA's stance that CBD is not a legal supplement, and
it's not yet clear if the DEA will change its current stance the legality of
CBD, with it being easier for farmers to grow, the supply of CBD may increase,
hopefully bringing down the high cost of CBD. It may also facilitate research
with CBD.
A prescription oral solution of cannabidiol (Epidiolex) was approved by
the FDA in June 2018 for the treatment of seizures associated with Dravet
syndrome and Lennox-Gastaut syndrome. It is the first FDA-approved drug that
contains a purified drug substance derived from marijuana, and the first FDA
approval of a drug for the treatment of patients with Dravet syndrome (FDA 2018). Epidiolex is
approved at a strength of 100 mg of CBD per milliliter and with a starting
dosage of 2.5 mg/kg twice daily, increasing to 5 mg/kg twice daily, and if
well-tolerated and needed, up to a maximum dosage of 10 mg/kg twice daily (Epidiolex Prescribing Information).
For a person weighing 70 kilograms (154 lbs), this would equal 350 mg to 1,400
mg of CBD per day.
Another condition for which CBD has been investigated as a new drug is cancer
pain (view a list here of completed,
ongoing, and planned studies with cannabidiol).
In Canada, cannabidiol is a controlled substance.
Products on the Market
Despite the fact that CBD cannot be legally sold in the U.S. as a dietary
supplement, many CBD products are available. Although it does not appear that
individuals have been prosecuted for purchasing these products for their own
use, the U.S. FDA has issued many warning letters to
companies selling products claiming to contain CBD and/or promoting such
products as treatments.
Tests of CBD products purchased at stores in Mississippi
showed that most labels did not accurately reflect actual CBD content. Among
five tested CBD oils, only Full Spectrum Hemp Oil (Functional
Remedies) contained its labeled amount of CBD, while Very Berry Syrup (www.Creating
BetterDays.com), CBD OIL (Green Roads), and Industrial
Hemp Tincture (East Tennessee Hemp Company) contained only 22%, 39%,
and 77%, respectively, and CBD Full Spectrum (Gold) (Hemp
Extracts) provided 157% of their labeled amounts. Among 14 CBD vape liquids,
two had their labeled amounts or slightly more, nine had just 0.06% to 54% of
their labeled amounts, and three that did not list specific amounts of CBD
contained synthetic cannabinoids (Gurley, J Diet Supp 2020).
The FDA also published the amounts of CBD, THC and other cannabis compounds it
found in products it tested in 2015 and 2016 (click here and select the
year to view). Most products contained very small concentrations of CBD —
similar to what is normally found in hemp oil (about 0.0025% CBD) while others
contained very large concentrations (25% to 35% CBD) yielding doses similar to
those used in clinical trials (typically 200 mg or more per day). Many of the
tested products did not contain the levels of CBD they claimed. The FDA
cautions that "Consumers should beware purchasing and using any such
products."
The reason why hemp oils would not be expected to contain much
CBD is that hemp oil is typically made from hemp seeds, which contain
little CBD (although some CBD may contaminate the surface of the
seeds). In fact, ConsumerLab.com has tested hempseed oils as part of its review of seed oil supplements (sources of omega-3 and omega-6 fatty
acids) and found those products to contain well under 1 mg
of CBD per serving. CBD is principally found in the flowers and, to a
lesser extent, the upper leaves of the hemp plant. A "CBD
oil" product is typically an oil, such as from hemp seed or other sources,
to which a CBD extract (from hemp flowers) has been added (Mead, Epilepsy & Behavior 2017).
In August 2019, a class action lawsuit was filed against
Just Brands USA Inc, the maker of JustCBD brand products. The
lawsuit alleges that independent laboratory testing (commissioned by the
plaintiff) revealed that some products contained substantially less CBD than
claimed on the label. JustCBD Liquid Honey Tincture, for example,
was found to contain 48.92 mg of CBD per bottle, rather than the 100 mg listed
on the label. JustCBD Apple Rings Gummies, labeled as containing
250 mg per jar, were found to contain no CBD. (Gaddis v. Just Brands USA, Inc. et
al).
At least one seller of CBD supplements to the public, PlusCBD LTD, appears to
claim that is not illegal to sell these products if they are derived from
"industrial" or "agricultural" hemp. Industrial hemp is
typically a larger plant with more stalk and less leaves and flowers than that
used to produce marijuana or CBD for medical use. It is grown for its fiber
(for textiles) and seeds (as food and oil), which would be very low in THC
(less than 0.3%) and CBD. It is true that the U.S. Drug Enforcement Agency (DEA)
has stated that CBD in trace amounts from cannabis stalk or
seeds is not a controlled substance, in contrast to CBD derived from cannabis
flower which is a controlled substance -- despite the compound being the same.
However, this does not seem to override the FDA's position that CBD cannot be
sold as dietary supplement. It would also seem difficult to obtain large
quantities of CBD from industrial hemp or cannabis stalk.
Many states now have medical
marijuana laws that permit products with high CBD content to be
sold by approved dispensaries and used by residents for medical purposes
recommended by a healthcare provider. In addition, several states without
medical marijuana laws allow products that are high in CBD (e.g., at least 5%,
10%, or 15% CBD) and low in THC (typically less than 0.3% to 0.9%) to be used
for specific medical purposes (typically intractable epilepsy) as approved or
recommended by a healthcare professional (See list of states on ProCon.org).
However, these state laws do not make the general sale of such products legal,
and some specifically require that the products be purchased out-of-state.
A synthetic form of CBD may be dangerous. The form, known as
4-CCB (or 4-cyano CUMYL-BUTINACA) is suspected in 52 cases of adverse
reactions, including altered mental status, seizures, shaking, confusion, loss
of consciousness, and hallucinations, associated with the use of CBD products
sold in Utah between 2017 and 2018. Testing confirmed the presence of this
synthetic compound in samples of "Yolo CBD oil" as well as other CBD
brands (not named) sold in the state. The tests also revealed these products
contained no actual CBD. The symptoms typically began within about 30 minutes
of exposure (Horth, MMWR Morb Mortal Wkly Rep
2018). Although this compound was not among those analyzed in
ConsumerLab's tests in this Review, it would seem unlikely to be in these products
since each was found to contain actual CBD.
Tests Performed
Considering the enormous variation that has been shown in the amounts of CBD in
marketed products and the fact that many don't accurately disclose their CBD
content, as noted above, ConsumerLab.com purchased a sampling of popular
products labeled to contain CBD, cannabinoids (other than THC), and/or hemp
extracts and tested them to determine the amounts of CBD and other cannabinoids
they contained, comparing these amounts to label expectations. Products
claiming to contain whole herbs or 250 mg or more of minerals per daily serving
or which were tested through CL's Quality Certification Program were
also tested for contamination with lead, cadmium and arsenic. See How Products Were Evaluated for
more details.
Products were compared on their label accuracy, quality, and cost.
What CL Found:
ConsumerLab found some significant changes in the CBD products
purchased in 2020 (see Results table and graphs below) compared
to those purchased in 2018/19:
·
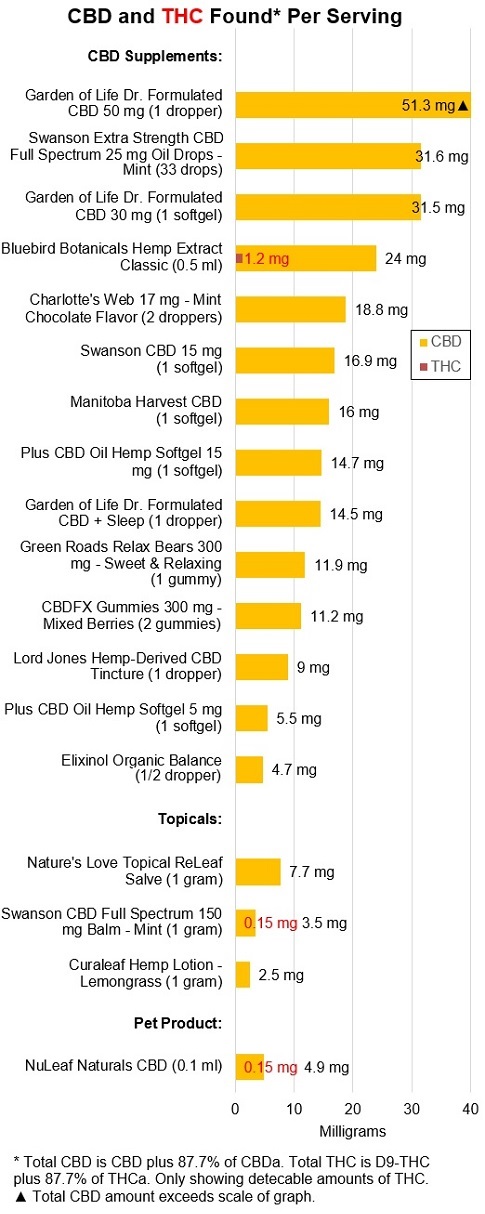
Products are generally formulated to contain greater
amounts of CBD than in the past. Amounts had ranged from 1.3 to 22.3 mg
per serving for oral products but now range from 4.7 mg to 51.3 mg, as shown
below.
·
Most products are now THC-free (i.e., no detectable THC). As also shown in
the graph above, only three products had detectable amounts of THC, with only
one, from Bluebird Botanicals, containing more than 1 mg per serving.
The move to THC-free products may have been driven by well-founded consumer
concerns of testing positive for THC on drug screens
when using CBD as well as potential effects of THC.
·
Prices have generally fallen and lower priced CBD products are
available. As shown in the graph below, the lowest cost to get 10 mg
of CBD was 24 cents. Although not inexpensive, this is a 70% decline from our
Review in 2018, when the lowest cost was 80 cents. In fact, six products in
this Review cost less than 80 cents per 10 mg. The drop in price may be due to
greater competition, especially from established supplement companies that have
entered the CBD market (such as Swanson and Garden of
Life), and a greater supply of CBD due to passage of the Farm Act in 2018
which legalized hemp production. Of course, there will always be premium-priced
products, such as Lord Jones Hemp-Derived CBD Tincture, which was
approximately 10 times as expensive as the lowest cost
Approved products, with a 10 mg dropperful of Lord Jones costing
$2.40 ($2.67 based on amount found).
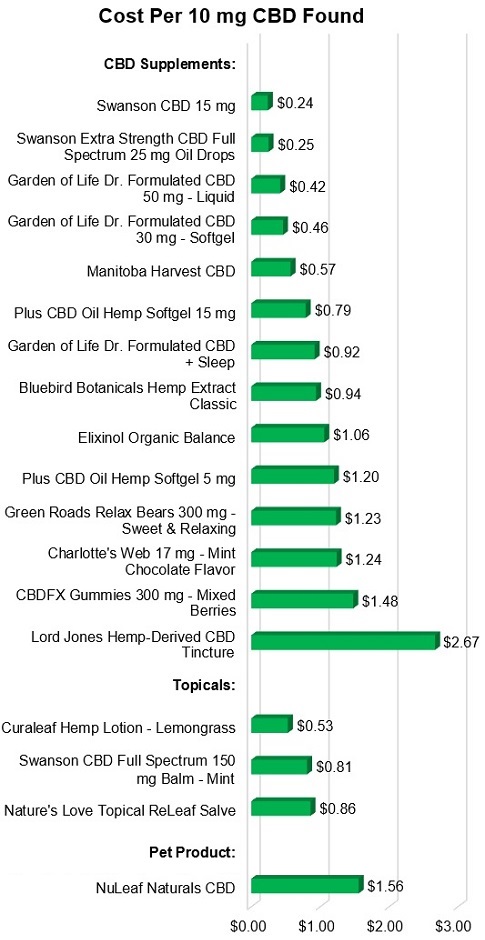
Every product contained
the amount of CBD listed on its label within an acceptable range and margin of
error (see How Products Were Evaluated). Note: Amounts of
CBD reported in this Review, include 87.7% of the amount of CBDa found as well
as the entire amount of CBD found. CBDa was not detectable in most products,
but it could be found in the following: Nature's Love Topical ReLeaf
Salve (2.24 mg per gram), NuLeaf Naturals CBD (0.007mg
per 2 drops), Bluebird Botanicals Hemp Extract Classic (0.225
mg per 0.5 mL), Plus CBD Oil Hemp Softgel 15 mg (0.07 mg per
softgel), and Plus CBD Oil Hemp Softgel 5 mg (4.03 mg per
softgel).
Several products did not specify amounts of CBD and, instead, listed amounts of
hemp extract, phytocannabinoids, or cannabinoids, all of which are more general
terms that include not only CBD but other compounds. Some companies likely
avoid the word "CBD" or "cannabidiol" on their product
labels out of concern for it triggering regulatory scrutiny. Nevertheless,
listed amounts of these broader ingredient names were found to closely resemble
the amounts of CBD found in each product -- although there is no guarantee that
this will always be the case. For example, nearly all of the "25 mg of
phytocannabinoids" in Bluebird Botanicals was CBD (24 mg
found) as was nearly all of Nature's Love 8.3 mg of hemp
extract (7.7 mg of CBD found). CBDFX gummies and Charlotte's
Web liquid actually contained somewhat more CBD than their claimed
amounts of cannabinoids.
In our tests in 2018, three products could not be approved: Two contained
significantly less CBD than listed and one contained more. This time around,
none of the products failed to contain their claimed or expected amounts of
CBD. In addition, all products tested for heavy metals were not found to be
contaminated. Consequently, all of the products selected for testing were
Approved.
Top Picks:
Among
products that passed all of the laboratory tests, CL identified its Top Picks,
representing a those providing superior value in addition to high quality.
CBD Oral Products for People
All-around: Swanson Extra Strength CBD Full Spectrum 25 mg Oil Drops — Mint. The
two Swanson products in this Review provided CBD at lowest
cost — about 24 to 25 cents per 10 mg, with Garden of Life products
being the next least costly, at 42 to 46 cents per 10 mg. Both Swanson products
are made from "full spectrum hemp extract" and clearly label their
CBD content. Although this oil is slightly more expensive per mg of CBD
than Swanson's 15 mg softgels, being a liquid it provides
flexibility of easily adjusting the dose up or down from the suggested 33 drops
(1 ml), which is listed as providing 25 mg of CBD (although we found 31.6 mg) —
so each drop provides nearly 1 mg of CBD. It also had no detectable THC, which
means that a full 33 drop dose would contain less than 0.0054 mg of THC, which
is far below the daily amount of 0.39 mg shown to occasionally trigger a
positive urine test for THC, as discussed above.
Low Dose (4-9 mg): We tested two
low-dose products, Elixinol (a liquid — 5 mg per ½ dropperful)
and Plus CBD Oil's 5 mg softgels. Although Elixinol is
a little less expensive (50 cents per 5 mg) than the Plus CBD Oil 5 mg
softgels (67 cents each), it is still about 5 times as expensive
as Swanson's oil (above), so it would make more sense to
purchase Swanson if you want an oil; you can just take a
smaller than suggested dose — about 5 or 6 drops would provide 5 mg for about
13 cents. However, if you want the convenience of a softgel at a low
dose, Plus CBD Oil's 5 mg softgels are our Top
Pick, although they are a more expensive source of CBD than Elixinol.
Moderate Dose (10-24
mg): Swanson CBD 15 mg. These softgels are the lowest-cost
source of CBD in this entire Review and each 15 mg softgel is 41 cents. As
with Swanson's liquid (above), the CBD in the softgels is from
"full spectrum hemp extract" and THC was undetectable. Be aware,
however, that neither Swanson product makes a statement about
being "THC-free" or below a particular level, so neither is
guaranteed to be low in THC even though we could not detect any.
High Dose (25 mg or
more): Swanson Extra Strength CBD Full Spectrum 25 mg Oil Drops — Mint. This is also
our all-around Top Pick for the reasons noted earlier. If you
prefer a softgel, our suggestion is Garden of Life's 30 mg softgels ($1.45 per
softgel), although it is about twice as expensive per mg of CBD as the Swanson
oil. Be aware that what is a "high dose" for CBD from these products
is still a relatively small dose compared to standard dosages of the
prescription CBD drug Epidiolex, which can easily be 500 mg to 1,000 mg per day
to treat children and teenagers with rare forms of epilepsy.
CBD Topical Balms & Lotions
Top Pick: Nature's Love Organic Topical ReLeaf Salve. This
product provided the highest concentration of CBD (7.7 mg per gram) among the
three topical products tested, although it is fairly expensive at 67 cents per
gram. A gram of Curaleaf Hemp Lotion — Lemongrass is much less
expensive, at 13 cents, but provides roughly one-third the amount of CBD (2.5
mg per gram). Swanson's balm provided a bit more CBD (3.5 mg
per gram) than Curaleaf's lotion but is more expensive at 28
cents per gram. ConsumerLab was informed by Nuume Organics (on 3/24/2021) that
Nature's Love was rebranded as Nuume Organics. Nuume Organics claims that the
formulation of this product is unchanged, however, we note that the ingredient
list on the Nuume Organics product is slightly different (listing specific
essential oils as opposed to listing only "essential oils") and we
have not tested product sold under the new brand name.
CBD for Pets
Top Pick: NuLeaf Naturals CBD Maximum Strength 240 mg CBD Per Bottle — This
was the only pet product selected for testing in the current Review but it
provides what it claims -- 4.8 mg of CBD per 2 drops for 77 cents. This is
relatively expensive (about 6 times the cost of CBD from Swanson's oil,
for example) but, unlike the oils for people tested in this Review, NuLeaf has
no added flavors, such as peppermint (which is in the oils from Swanson
and Garden of Life). It is difficult to know how such flavors would be
tolerated by a pet. Another possible option to reduce cost would be to open
a Swanson 15 mg softgel (41 cents) and use an appropriate
amount of oil, since that oil is not flavored. Each Swanson softgel
contains 15 mg of CBD, so one third of its contents is comparable to 2 drops
of NuLeaf's oil.
Test Results by Product:
The
table below lists test results and information for 18 products containing CBD
grouped first by those for people, then topicals and then for pets. Products
are shown alphabetically within each category. ConsumerLab.com selected ten of
these products based on reader suggestions and popularity in the marketplace.
Eight other products (each indicated with a CL flask) were tested at the request
of their manufacturer/distributor through CL's Quality Certification Program and are included
for having passed testing.
Also shown below for each product is the claimed type and amount
of hemp ingredient and cannabinoids per serving or unit, the serving size
recommended on the label, the amount of CBD, THC, and other cannabinoids found,
dietary designations if claimed on the label (i.e. Kosher, Non-GMO) and
ingredient and cost comparisons. Check marks in the table indicate that a
product passed ConsumerLab.com's quality criteria for that attribute (see Passing Score). The full list of ingredients for each product is found in the
last column.
Results of
ConsumerLab.com Testing of CBD OIL PRODUCTS
(Click arrows or swipe left or right to see all columns)
Product Name
(Suggested Serving on Label)
Claimed Amount of Hemp Extract and/or CBD
Cannabinoids Findings
Suggested Serving on Label
Pill Sizeⓘ
Heavy Metalsⓘ
Cost for Suggested Serving
[Cost Per 10 mg CBDⓘ]
Priced
Notable Features
Full List of Ingredients Per Serving
CBD Supplements (Gummies, Liquids &
Softgels):
APPROVED
Bluebird Botanicals Hemp Extract Classic
Dist. by Bluebird Botanicals
Serving: 0.5 ml
25 mg full-spectrum cannabinoids
Found:
CBDⓘ:
24 mg
THCⓘ:
1.2 mg
% THCⓘ: 0.12% (claims
<0.3% Δ9-THC)
✔
THC/CBD ratio: 2.5%
✔
Other cannabinoidsⓘ:
0.94 mg
Total cannabinoids: 25.9 mg
✔
5% of oil is CBDⓘ
Do not exceed 1 ml (2 servings) per day.
Liquid from bottle
Heavy metals: Pass
$2.25/0.5 ml
[$0.94 based on amount found]
$44.95/0.33 fl oz [10 ml] bottle (approx. 20 servings)
This product contains hemp, which has <0.3%
Delta 9-THC.
0.5 ml
Full Spectrum Cannabidiols 25 mg Per Serving.
Other Ingredients: None Listed.
APPROVED
CBDFX® Gummies 300 mg - Mixed Berries
Dist. by CBDFX
Serving: 2 gummies
12 mg hemp extract
10 mg cannabinoids
Found:
CBDⓘ:
11.2 mg
No THC detectedⓘ
(claims <0.2% THC)
✔
Other cannabinoidsⓘ:
0 mg
Total cannabinoids: 11.2
✔
0.3% of gummy is CBDⓘ
No directions on label
Large circular gummy
Heavy metals: NA
$1.67/2 gummies
[$1.48 based on amount found]
$49.99/60 gummies
This product contains less than 0.2% THC. Vegan.
Non-GMO. No Corn Syrup.
2 gummies
Calories 30, Carbohydrates 7 g, Sugar (As organic cane sugar) [Includes 1 g
added sugars] 1 g, Hemp Extract (Aerial Parts) 12 mg, Cannabinoids 10 mg.
Other Ingredients: Organic Tapioca Syrup, Organic Cane Sugar, Purified Water,
Pectin, Tri Sodium Citrate, Citric Acid, Tomato Lycopene (for color), Natural
Flavor.
APPROVED
Charlotte's Web™ 17 mg - Mint Chocolate Flavor
Dist. by Charlotte's Web, Inc.
Serving: 2 droppers [1 ml]
28 mg hemp extract
17 mg phyto-
cannabinoids
Found:
CBDⓘ:
18.8 mg
No THC detectedⓘ
Other cannabinoidsⓘ:
1.3 mg
Total cannabinoids: 20.1 mg
✔
2% of oil is CBDⓘ
Adults: Take Two Full Droppers (1 ml) Up To Two
Times Daily.
Liquid from bottle
Heavy metals: NA
$2.33/2 droppers
[$1.24 based on amount found]
$69.99/1 fl oz [30 ml] bottle (approx. 30 servings)
Kosher. Gluten Free. Non GMO. 100% Vegan.
Precaution: Contains Coconut.
2 droppers
Calories 10, Total Fat 1 g, Hemp Extract (aerial parts) [Minimum naturally
occurring total phyto-
cannabinoids, 17 mg] 28 mg.
Other Ingredients: Fractionated Coconut Oil, Organic Mint Chocolate (Organic
Sunflower Oil, Natural Flavors).
APPROVED
Elixinol Organic Balance
Dist. by Elixinol LLC
Serving: 1/2 dropper [0.5 ml]
5 mg CBD
Found:
CBDⓘ:
4.7 mg
✔
No THC detectedⓘ
(claims <0.3% THC)
✔
Other cannabinoidsⓘ:
0.39 mg
1% of oil is CBDⓘ
Take twice daily with food or as needed.
Liquid from bottle
Heavy metals: NA
$0.50/0.5 dropper
[$1.00 based on amount listed]
[$1.06 based on amount found]
$29.99/1 fl oz [30 ml] bottle (approx. 60 servings)
This product contains less than 0.3% THC in
accordance to USDA regulations. USDA Organic seal.
1/2 dropper
Cannabidiol (CBD) 5 mg.
Other Ingredients: Organic MCT Coconut Oil, Organic Whole Plant Hemp Extract,
Organic Copaiba Oil.
APPROVED
Garden of Life® Dr. Formulated CBD + Sleep![]()
Dist. by Garden of Life LLC
Serving: 1 dropperful [1 ml]
944 mg hemp extract
15 mg CBD
Found:
CBDⓘ:
14.5 mg
✔
No THC detectedⓘ
(claims THC free)
✔
Other cannabinoidsⓘ:
0.16 mg
1.5% of oil is CBDⓘ
Adults take 1 dropperful (1 ml) at bedtime.
Liquid from bottle
Heavy metals: Pass
$1.33/dropper
[$0.89 based on amount listed]
[$0.92 based on amount found]
$39.99/1 fl oz [30 ml] bottle (approx. 30 servings)
1 dropperful
Vitamin D 20 mcg (800 IU), entourage effect
blend 4.05 mg.
igen™ Non-GMO Tested seal. Labdoor Certified THC Free seal. Gluten Free.
Precaution: Contains: Tree nuts (coconut).
1 dropperful
Vitamin D (as D3) 20 mcg (800 IU), Broad Spectrum Hemp Extract Blend [Hemp
Extract (whole plant) in Organic MCT Oil (from coconut) [15 mg Cannabidiol
(CBD)]] 944 mg, Entourage Effect Blend [Essence of Organic Lemon Balm (leaf)
Oil, Essence of Organic Orange (peel) Oil, Essence of Organic Lavandin (flower
& stem) Oil, Essence of Organic Chamomile (leaf) Oil, more...
APPROVED
Garden of Life® Dr. Formulated CBD 30 mg -
Softgel![]()
Dist. by Garden of Life LLC
Serving: 1 softgel
341 mg hemp extract
30 mg CBD
Found:
CBDⓘ:
31.5 mg
✔
No THC detectedⓘ
(claims THC free)
Other cannabinoidsⓘ:
0.12 mg
7.8% of oil is CBDⓘ
Adults take 1 or more softgels daily as desired.
Medium/large softgel
Heavy metals: Pass
$1.45/softgel
[$0.48 based on amount listed]
[$0.46 based on amount found]
$43.39/30 softgels
1 softgel
Entourage effect blend 73 mg
igen™ Non-GMO Tested seal. Labdoor Certified THC Free seal. Vegan. Gluten
Free.
Precaution: Contains: Tree nuts (coconut).
1 softgel
Broad Spectrum Hemp Extract Blend [Hemp Extract (whole plant) in Organic MCT
Oil (from coconut) [30 mg Cannabidiol (CBD)]] 341 mg, Entourage Effect Blend
[Essence of Pure Clove (flower) Oil, Essence of Pure Rosemary (leaf) Oil,
Essence of Pure Pepper (fruit) Oil] 73 mg.
Other Ingredients: Non-GMO PlantGel™ Softgel.
APPROVED
Garden of Life® Dr. Formulated CBD 50 mg -
Liquid![]()
Dist. by Garden of Life LLC
Serving: 1 dropperful [1 ml]
946 mg hemp extract
50 mg CBD
Found:
CBDⓘ:
51.3 mg
✔
No THC detectedⓘ
(claims THC free)
Other cannabinoidsⓘ:
0.32 mg
5.2% of oil is CBDⓘ
Adults take 1 dropperful (1 ml) 1 or more times
daily as desired.
Liquid from bottle
Heavy metals: Pass
$2.17/dropperful
[$0.43 based on amount listed]
[$0.42 based on amount found]
$65.09/1 fl oz [30 ml] bottle (approx. 30 servings)
1 dropperful
Entourage effect blend 1.8 mg
igen™ Non-GMO Tested seal. Labdoor Certified THC Free seal. Vegan. Gluten
Free.
Precaution: Contains: Tree nuts (coconut).
1 dropperful
Broad Spectrum Hemp Extract Blend [Hemp Extract (whole pant) in Organic MCT Oil
(from coconut) [50 mg Cannabidiol (CBD)]] 946 mg, Entourage Effect Blend
[Essence of Organic Peppermint (flower, leaf, stem) Oil, Essence of Organic
Orange (peel) Oil, Essence of Organic Lavandin (flower & stem) Oil, Essence
of Organic Frankincense (resin) Oil] 1.8 mg, more...
APPROVED
Green Roads® Relax Bears 300 mg - Sweet &
Relaxing
Dist. by Green Roads of Florida LLC
Serving: 1 gummy
10 mg CBD
Found:
CBDⓘ:
11.9 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
0 mg
0.4% of gummy is CBDⓘ
No directions on label
Large bear-shaped gummy
Heavy metals: NA
$1.47/gummy
[$1.47 based on amount listed]
[$1.23 based on amount found]
$43.99/30 gummies
None.
1 gummy
Calories 10, Total Fat 0 g, Saturated Fat 0 g, Trans Fat 0 g, Cholesterol 0 mg,
Sodium 0 mg, Total Carbohydrate 3 g, Dietary Fiber 0 g, Total Sugars [Includes
0 g Added Sugar] 2 g, Protein 0 g.
Ingredients: Corn Syrup, Sugar (from beets), Water, Gelatin, Citric Acid,
Natural and Artificial Flavors, Yellow 5, Yellow 6, Blue 1, 300 mg Hemp-Derived
Cannabinoid Extract.
APPROVED
Lord Jones Hemp-Derived CBD Tincture - Naturally
Flavored Peppermint
Dist. by Redwood Wellness, LLC
Serving: 1 full dropper [1.2 ml]
10 mg CBD
Found:
CBDⓘ:
9 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
0.072 mg
0.8% of oil is CBDⓘ
Place one full dropper in mouth for 60 seconds
and swallow. Repeat as needed.
Liquid from bottle
Heavy metals: NA
$2.40/full dropper
[$2.40 based on amount listed]
[$2.67 based on amount found]
$60.00/1 fl oz [30 ml] bottle (approx. 25 servings)
None.
1 full dropper [1.2 ml]
Calories 10, Calories From Fat 10, Total Fat 1 g, Cannabidiol (CBD) [from broad
spectrum hemp extract] 10 mg.
Other Ingredients: Grape Seed Oil, Hemp Seed Oil, Peppermint Oil, Stevia Leaf
Extract.
APPROVED
Manitoba Harvest® CBD
Dist. by Manitoba Harvest USA LLC
Serving: 1 softgel
17 mg hemp extract
15 mg CBD
Found:
CBDⓘ:
16 mg
✔
No THC detectedⓘ
(claims <0.01% THC)
Other cannabinoidsⓘ:
0 mg
4.4% of oil is CBDⓘ
Adults, take 1 softgel 1-2 times daily. Increase
if needed.
Medium/large softgel
Heavy metals: NA
$0.92/softgel
[$0.61 based on amount listed]
[$0.57 based on amount found]
$54.99/60 softgels
THC Free (Less Than 0.01% THC). Vegan. Non GMO,
Gluten Free.
1 softgel
Broad Spectrum Hemp Extract (Cannabis sativa L.) (aerial parts)
[Cannabidiol (CBD) 15 mg] 17 mg.
Other Ingredients: Organic Hemp Seed Oil, Modified Cornstarch, Glycerin,
Carrageenan, Purified Water.
APPROVED
Plus CBD Oil Hemp Softgel 15 mg![]()
Dist. by CV Sciences, Inc.
Serving: 1 softgel
15 mg CBD
(in 60 mg hemp oil)
Found:
CBDⓘ:
14.7 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
1.1 mg
6.1% of oil is CBDⓘ
As a dietary supplement, take 1 softgel.
Medium/large softgel
Heavy metals: Pass
$1.17/softgel
[$0.78 based on amount listed]
[$0.79 based on amount found]
$69.99/60 softgels
Non GMO. Gluten-Free.
1 softgel
Hemp Oil (Aerial Plant Parts) (Cannabidiol (CBD) 15 mg) 60 mg.
Other Ingredients: Extra Virgin Olive Oil, Vegetable Softgel (Vegetable
Cellulose, Water), Silica.
APPROVED
Top Pick
for low dose (4-9 mg)
Plus CBD Oil Hemp Softgel 5 mg![]()
Dist. by CV Sciences, Inc.
Serving: 1 softgel
5 mg CBD+CBDa
(in 60 mg hemp oil)
Found:
CBDⓘ:
5.5 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
0.29 mg
2.3% of oil is CBDⓘ
Take 1 softgel.
Medium/large softgel
Heavy metals: Pass
$0.67/softgel
[$1.20 based on amount found]
$39.99/60 softgels
RAW Formula. Non GMO. Gluten-Free.
1 softgel
Hemp Oil (Aerial Plant Parts) (Cannabidiolic Acid/ Cannabidiol (CBDa/CBD) 5 mg)
60 mg.
Other Ingredients: Extra Virgin Olive Oil, Vegetable Softgel (Vegetable
Cellulose, Water), Silica.
APPROVED
Top Pick
for moderate dose (10-24 mg)
Dist. by Swanson Health Products
Serving: 1 softgel
20.6 mg hemp extract
15 mg CBD
Found:
CBDⓘ:
16.9 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
0.17 mg
13.1% of oil is CBDⓘ
As a dietary supplement, take one softgel per
day with water.
Medium softgel
Heavy metals: Pass
$0.41/softgel
[$0.27 based on amount listed]
[$0.24 based on amount found]
$24.69/60 softgels
Non-GMO. Gluten Free. Vegan.
1 softgel
Full Spectrum Hemp Extract (Cannabis sativa L.) (aerial parts)
[Cannabidiol (CBD) 15 mg] 20.6 mg.
Ingredients: Vegetarian softgel (modified food starch, glycerin, carrageenan,
water, annatto), sunflower oil.
APPROVED
Top Pick
for all-around and high dose (25 mg or more)
Swanson Extra Strength CBD Full Spectrum 25 mg
Oil Drops - Mint![]()
Dist. by Swanson Health Products
Serving: 33 drops [1 ml]
41.67 mg hemp extract
25 mg CBD
Found:
CBDⓘ:
31.6 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
2.4 mg
6.5% of oil is CBDⓘ
As a dietary supplement, take 33 drops (1 ml)
per day.
Liquid from bottle
Heavy metals: Pass
$0.79/33 drops
[$0.32 based on amount listed]
[$0.25 based on amount found]
$47.50/2 fl oz [60 ml] bottle (approx. 60 servings)
Non-GMO. Vegan. Gluten Free.
Precaution: Contains tree nuts (coconut).
33 drops
Calories 10, Total Fat 1 g, Saturated Fat 0.8 g, Full Spectrum Hemp Extract (Cannabis
sativa L.) (Flowers and Leaves) [Cannabidiol (CBD) 25 mg] 41.67 mg.
Other Ingredients: Organic coconut MCT oil, natural flavors (peppermint), mixed
tocopherols, luo han guo.
Topicals (Balm, Lotion & Salve):
APPROVED
Curaleaf™ Hemp Lotion - Lemongrass
Dist. by Curaleaf KY, Inc.
Serving: 1 g
2.6 mg CBD
Found:
CBDⓘ:
2.5 mg
✔
No THC detectedⓘ
Other cannabinoidsⓘ:
0 mg
0.2% of lotion is CBDⓘ
Massage into skin until thoroughly absorbed. Use
carefully on the face. Avoid eyes, nose, mouth and ears. Repeat as desired.
Most effective when applied to clean, dry skin directly after a shower or bath.
Lotion in jar
Heavy metals: NA
$0.13/gram
[$0.50 based on amount listed]
[$0.53 based on amount found]
$14.99/4 oz [113.4 g] jar
Free From: wheat, soy, nuts, eggs, dairy.
Contains 300 mg Total CBD [Per Container].
Ingredients: Aqua, Butyrospemum Parkii (Shea) Butter, Cetearyl Alcohol (and)
Cetearyl Glucoside, Tapioca Starch Modified, Organic Aloe Barbadensis (aloe
Vera) Leaf Juice, Caprylic Capric Triglyceride, Aroma (Lemongrass), Helianthus
Annuus (Sunflower) Seed Oil, Organic Argania Spinosa (Argan) Kernel Oil, more...
APPROVED
Top Pick
for CBD topical balms & lotions
Nature's Love™ Topical ReLeaf Salveⓘ
Mfd. by Nature's Love
Serving: 1 g
8.3 mg hemp extract
Found:
CBDⓘ:
7.7 mg
No THC detectedⓘ
Other cannabinoidsⓘ:
0.43 mg
0.8% of salve is CBDⓘ
For external use only. Massage deeply into skin.
Salve in jar
Heavy metals: NA
$0.67/gram
[$0.86 based on amount found]
$39.99/2 fl oz [60 ml] jar
USDA Organic seal.
Precaution: Allergen Warning: This product contains Coconut and
Tree Nut Oils.
Hemp Extract 500 mg [Per Container].
Ingredients: Organic Virgin Coconut Oil, Organic Shea Butter, Organic Beeswax,
Organic Proprietary Cultivars Hemp Extract, Organic Essential Oils.
APPROVED
Swanson CBD Full Spectrum 150 mg Balm - Mint![]()
Dist. by Swanson Health Products
Serving: 1 g
2.6 mg CBD
Found:
CBDⓘ:
3.5 mg
✔
THCⓘ:
0.15 mg
% THCⓘ: 0.02%
THC/CBD ratio: 4.4%
✔
Other cannabinoidsⓘ:
0.19 mg
0.3% of balm is CBDⓘ
Apply to skin as needed. For External Use Only.
Balm in jar
Heavy metals: Pass
$0.28/gram
[$0.81 based on amount found]
$15.99/2 oz [57 g] bottle
None.
Ingredients: Helianthus annuus (sunflower) seed
oil, Cocos nucifera (coconut oil), beeswax, Olea
europaea (olive) oil, Cannabis sativa (hemp), Mentha
piperita (peppermint) oil, cetearyl glucoside (and) cetearyl alcohol.
Pet Products:
APPROVED
Top Pick
for CBD for pets
NuLeaf Naturals CBD Maximum Strength - 240 mg
CBD per bottle
Dist. by NuLeaf Naturals
Serving: 2 drops (0.1 ml)
4.8 mg CBD
Found:
CBDⓘ:
4.9 mg
THCⓘ:
0.15 mg
% THCⓘ: 1.4%
THC/CBD ratio: 3.1%
✔
Other cannabinoidsⓘ:
0.43 mg
Total cannabinoids: 5.5 mg
✔
5.2% of oil is CBDⓘ
Give 2-4 drops per 25 lbs.
Liquid from bottle
Heavy metals: Pass
$0.77/2 drops
[$1.60 based on amount claimed]
[$1.56 based on amount found]
$38.50/0.17 fl oz [5 ml] bottle (approx. 50 servings)
No GMOs.
2 drops = 4.8 mg Cannabidiol.
Other Ingredients: None Listed.
Unless otherwise noted, information about the
products listed above is based on the samples purchased by ConsumerLab.com (CL)
for this Product Review. Manufacturers may change ingredients and label
information at any time, so be sure to check labels carefully when evaluating
the products you use or buy. If a product's ingredients differ from what is
listed above, it may not necessarily be of the same quality as what was tested.
The information contained in this report is
based on the compilation and review of information from product labeling and
analytic testing. CL applies what it believes to be the most appropriate
testing methods and standards. The information in this report does not reflect
the opinion or recommendation of CL, its officers or employees. CL cannot
assure the accuracy of information.
Copyright ConsumerLab.com, LLC, 2021 All
rights reserved. Not to be reproduced, excerpted, or cited in any fashion
without the express written permission of ConsumerLab.com LLC
ConsumerTips™:
Read
Labels Carefully
As noted earlier, hemp oil would not be expected to contain
much CBD, while hemp extract or products that specifically
list CBD as an ingredient would be expected to contain CBD. Ideally, look for a
product that lists the amount of CBD per serving (not just per entire bottle).
If a product lists "cannabinoids" it may contain some CBD but you
won't know how much.
Dosing:
Most of the research with CBD has involved high doses (several hundred
milligrams daily). This is much more than you'll typically get from products
being sold on the market. Here's what's been shown to work in preliminary
clinical studies, as discussed in more detail in the What It Does section:
·
Anxiety (relating to public speaking): 300 mg to 600 mg of
CBD daily
·
Insomnia: 160 mg of CBD 30 minutes before bedtime to increase sleep
time (but will not decrease the amount of time it takes to fall asleep)
·
Reduction in seizures: 20 mg of CBD per kilogram of body weight
daily (e.g., for a 70 kg adult, this would be 1,400 mg of CBD)
·
Schizophrenia: 1,000 mg of CBD daily
·
For dogs with osteoarthritis: 2 mg per kilogram of bodyweight, twice
daily. (e.g., for a 20 lb. dog: 18 mg of CBD in the morning and again at
night).
How to Take:
CBD is fat-soluble (i.e., lipophilic) and taking CBD with, or shortly,
after a meal containing fats can dramatically increase its bioavailability which
otherwise may be as low as 6% (Devinksy, Epilepsia 2014). A 5-fold increase
in blood levels of CBD occurred when an oral solution of CBD (in an alcohol and
oil base) was taken with a high-fat/high-calorie meal (i.e., a large meal containing
fats) rather than on an empty stomach (FDA - Epidiolex labeling revised
6/2018). Similarly, another small study
found a 4-fold increase in blood levels of CBD when a single dose of CBD (200
to 300 mg of CBD in capsules as a 99% purified CBD extract, Vireo
Health Violet) was taken with a high-fat meal (500 to 600 Calories from
fat) rather than on an empty stomach. In addition, blood levels temporarily
reached a maximum level 14 times higher and the half-life of
CBD in the blood was extended to 39 hours from 24 hours when CBD was taken with
food rather than on an empty stomach (although it's not clear if these effects
would be as pronounced with lower doses of CBD) (Birnbaum, Epilepsia 2019).
Special formulations of CBD exist that may enhance absorption
of CBD, possibly reducing the need for it to be taken with a meal containing fat.
A small, company-funded study in healthy men and women in Germany found that,
taken on an empty stomach, absorption of a single 25 mg dose of CBD from hemp
extract formulated with an absorption enhancer (VESIsorb) increased
blood levels of CBD 2.9 times as much as the same extract in a small amount of
oil (medium-chain triglycerides), although, over 24 hours, the increase was
just 1.7 times that of the CBD in oil (Knaub, Molecules 2019). Unfortunately, these
increases are not as great as that achieved by just taking CBD with a
fat-containing meal, as discussed above. (VESIsorb is a
"nano-colloid" that includes triglycerides, polyglycerol esters and
polysorbate 80 and is also used in some CoQ10 and resveratrol formulations
to increase absorption).
A liposomal formulation of CBD produced using sunflower lecithin (phosphatidyl
choline) was shown to increase the bioavailability of a 10 mg dose of CBD by
17-fold one hour after consumption compared to an unformulated CBD. In fact, CBD
was undetectable in two of the five volunteers taking unformulated CBD.
However, the CBD was taken without food and on an empty stomach (Verrico, Pain 2020).
After taking CBD orally, maximal blood levels are reached within about 2.5 to 5
hours. CBD remains in the blood for a fairly long time — studies have shown its
half-life (i.e., the time for blood levels to fall by half) to be 18 to 32
hours (Devinksy, Epilepsia 2014)
to as long as 56 to 61 hours after 7 days of repeated high-dosing (FDA - Epidiolex labeling revised
6/2018).
Does CBD convert to THC in the body?
Although some preliminary laboratory studies suggested that CBD may convert to
THC during digestion (Merrick, Cannabis Cannabinoid Res
2016), a clinical trial in people showed that no THC was
detected in the blood up to six hours after consuming a large dose of CBD (300
mg of a 99.5% pure CBD oral solution in corn oil) both with and without food.
In addition, none of the participants experienced any psychoactive effects
associated with THC (Crippa, Cannabis Cannabinoid Res 2019).
Concerns and Cautions:
CBD can
cause side effects and interact with certain medications and conditions, although
these effects have typically been reported only with very high daily intake,
i.e., hundreds of milligrams daily.
·
High daily doses of CBD (20 mg per kg of body weight, i.e.,
hundreds of milligrams) may cause decreased appetite, diarrhea,
vomiting, fatigue, fever, somnolence, and abnormal results on liver-function
tests (Devinsky, New Eng J Med 2017; Thiele, Lancet 2018).
Elevated liver enzymes have been shown to occur in 8% and 16% of patients
given, respectively, 10 and 20 mg of CBD per kg of body weight daily, and it
is, therefore, recommended that high-dose CBD should be used with caution in
people with pre-existing liver disease and when taking other drugs that can
adversely affect the liver, such as antiepileptics, antipsychotics,
acetaminophen, certain antibiotics (amoxicillin and nitrofurantoin),
antifungals, and verapamil (Brown, J Clin Med 2019).
Side effects at very high dosage are common: A dose of 25 mg
per kg resulted in adverse events in 80.8% of children (treated for epilepsy),
with decreased appetite, diarrhea, and weight loss being the most common
events. Weight loss emerged only after several months on treatment, was
clinically significant in 30.7% of patients, and resolved with dose reduction
or treatment cessation (Sands, CNS Drugs 2018).
·
CBD may lower blood pressure and increase heart rate. A small study among
young, healthy men (average age 23) found that, compared to placebo, a single,
600 mg dose of CBD decreased resting average systolic blood pressure (by 6 mm
Hg) and increased heart rate by about 10 beats per minute (bpm) approximately
one hour after taking; the same dose of CBD also lowered systolic blood
pressure (by 5 mm Hg) and increased heart rate (by 10 bpm) during exercise (Jadoon, JCI Insight 2017). Drops in blood pressure (of 10 to 20 mm Hg) upon
standing were reported in each of five patients with movement disorders given
CBD (100 mg to 500 mg) for several weeks. Two patients also reported lightheadedness.
A worsening of symptoms of Parkinson's disease was also noted.
In addition, dry mouth occurred in two of the five patients (Consroe, Int J Neurosci 1986). Dry mouth
(which also occurs with marijuana use) may be due to inhibition of cells in the
salivary glands that contain cannabinoid receptors CB1 and CB2 (Prestifilippo, Exp Biol Med 2006), and CBD can
interact with such receptors (Pertwee, Br J Pharmacol 2008).
·
Low-dose, full spectrum hemp extracts appear to be generally
safe and well-tolerated, based on the amount and type of adverse events
reported with PlusCBD products in 2018 through 2019. The most common adverse
event reported with oral use was abdominal discomfort, representing
24% of all adverse events reported from 2018 through 2019. This was followed
by headache (7%), hypersensitivity (6%),
and nausea (6%). Out of 1,334 reports, only two were
considered serious but may not have been due to the product. With topical products, hypersensitivity, dermatitis,
and rash were most common, although there were only 95 reports
in total. During the reporting period, 5 million units were sold, indicating
that only 0.03% of sales resulted in an adverse event reported to the company (Schmitz, J Diet Supp 2020).
·
Two days after starting to supplement with CBD, a red, pustular
bilateral rash occurred on the arms, underarms, buttocks and
groin of a 63-year-old man with a history of plaque psoriasis. The rash
improved within four days of stopping CBD, and completely cleared several weeks
later. This rash was characterized as a pustular drug eruption — which
typically appears within 48 hours of exposure to a drug to which an individual
is highly sensitive (Pettit, Dermatitis 2018).
(ConsumerLab has contacted the author of this report for more details about the
CBD product and dose and will post this information if received).
·
CBD should be used with caution with sedative and
sleep-inducing medications, as it may enhance their effects. CBD could also
potentially increase the effects of herbs and supplements that have a
sedating effect, such as melatonin, valerian, SAMe, ashwagandha and
others, although there do not appear to be any published reports of this
occurring.
·
Be aware that CBD may interact with other medications that,
like itself, are metabolized in the body by the cytochrome p450 enzymes CYP3A4
and CYP2C19. By competing for these enzymes, CBD can reduce the metabolism of
such drugs, raising their blood levels and possibly requiring downward
adjustment of their doses. For example, several hundred milligrams of
CBD daily in epilepsy trials have caused sizable increases in levels of
the epilepsy medications clobazam, valproic acid, levetiracetam,
felbamate, lamotrigine, and zonisamide (U.S Department of Health; Yamaori, Life Sci 2011; Iffland, Cannabis Cannabinoid Res 2017). Adding 50 mg to 300 mg of CBD per day to treatment with the
epilepsy drug brivaracetam was found to increase blood levels of brivaracetam
by as much as 95% to 280% (Klotz, Epilepsia 2019).
For the same reason, CBD may increase the blood-thinning
effects of warfarin (Coumadin, Jantoven). This was observed in a man
with post-stroke epilepsy taking warfarin: His INR (a measure of how long it
takes blood to clot) began increasing several weeks after starting CBD (Epidiolex,
Greenwich Biosciences, Inc.) for his seizures. His CBD dosing started with
several hundred milligrams daily and increased to over 1,000 mg, at which point
his warfarin dose had been reduced by approximately 30% (Grayson, Epilepsy Behav Case Rep 2017). Similarly,
a dose-dependent increase in INR was reported in a 46-year-old man taking
warfarin (80 mg daily) after he began taking Epidiolex (805 mg/day increased to
over 3,000 mg/day over several weeks) to help control epileptic seizures. He
required a 20% reduction in warfarin dosage in order maintain INR within goal
range while continuing Epidiolex (Cortopassi, Am J Health Syst Pharm 2020).
These effects are dose-dependent, and it's possible
that lower doses of CBD (single digits or tens of milligrams daily), as in many
products in this Review, will have more modest effects.
Nevertheless, use CBD with caution if you take medications
metabolized by the enzyme affected by it. Such medications include macrolide
antibiotics such as clarithromycin and azithromycin (Zithromax), cyclosporine
(Sandimmune), sildenafil (Viagra), antihistamines, Haloperidol (Haldol),
certain statin medications such as atorvastatin (Lipitor) and simvastatin
(Zocor), testosterone, progesterone, nifedipine (Procardia XL), metoprolol
(Lopressor, Toprol XL), the proton pump inhibitor drug omeprazole (Zegerid,
Prilosec OTC) and the antipsychotic drug risperidone (Risperdal), ondansetron
(Zofran), paroxetine (Paxil), flecainide (Tambocor) and others, such as fluoxetine (Prozac) and fluvoxamine (Luvox), clopidogrel
(Plavix), propranolol (Inderal LA), carisoprodol (Soma), cyclophosphamide
(Cytoxan), fluconazole (Diflucan), efavirenz (Sustiva, Stocrin), celecoxib
(Celebrex), naproxen (Aleve), sulfonylureas (Amaryl, Glynase), losartan
(Cozaar), rosuvastatin (Crestor), valsartan (Diovan), enzalutamide (Xtandi) (Brown, J Clin Med 2019). As mentioned above, CBD should also be used with
caution when taking medications with the potential to cause liver damage, such
as acetaminophen.
·
There is also evidence that very high doses of
CBD can inhibit the cytochrome p450 enzyme CYP1A2 and, therefore, potentially
increase blood levels of caffeine and other drugs that are metabolized by this
enzyme. A study showed that when people taking 1,500 mg of CBD were given
caffeine, the amount of caffeine in their blood was nearly double the amount
that occurred when caffeine was given without CBD (Thai, Clin Pharmacol Drug Dev 2021). Other
drugs metabolized by this enzyme include clozapine (Clozaril), Fluvoxamine
(Luvox), frovatriptan (Frova) mirtazapine (Remeron) and zolmitriptan (Zomig).
·
A small study in healthy men found that a single dose (400 mg)
of antifungal drug ketoconazole (Nizoral, Xolegel, Extina)
increased blood levels of CBD, while a single dose (600 mg) of the
antibiotic rifampicin (Rifadin) decreased blood levels of CBD
(Stott, Springerplus 2013).
·
A dangerous synthetic cannabinoid (AB-FUBINACA) has
been found in some CBD products, including e-cigarette fluid and incense.
AB-FUBINACA-related compounds have been linked to several deaths (Shanks, J Anal Toxicol 2016; Lam, Clin Toxicol 2017).
It was also detected in a CBD oil purchased from what a consumer believed to be
a "reputable online distributor from Colorado." The discovery was
made after an 8-year-old boy was given the oil for nine days to help treat a
seizure disorder but, instead, experienced a sudden increase in the frequency
of seizures as well as agitation, delirium, dilated pupils and rapid heartbeat.
The CBD oil (identity not disclosed in report) was found to contain CBD as well
as AB-FUBINACA — which is known to cause these symptoms (Rianprakaisang, Clin Toxicol 2019).
(ConsumerLab may screen for synthetic cannabinoids in future tests).
·
CBD supplements can contain enough THC to cause a positive
drug test for marijuana (using a 50 ng/mL of urine limit). This has
been demonstrated in several small studies. In one, one of seven adults tested
positive at a dose of just 0.39 mg of THC per day (Gustafson, Clin Chem 2003).
In the other study, one of 15 people had a positive drug test at a dose of 0.6
mg of THC per day (Leson J Anal Toxicol 2001). In
a third study, 7 out of 14 people tested positive after taking a CBD liquid
providing approximately 0.8 mg of THC daily for 4 weeks (the liquid was taken
sublingually in three divided doses each day, with each dose containing about
10 mg of CBD from a "full spectrum" hemp extract) (Dahlgren, JAMA Psych 2021). There
is likely much person-to-person variability in these results due to differences
in how people absorb and metabolize THC. Note that higher doses of CBD from
hemp extracts will likely provide higher amounts of THC. THC (like CBD) is
fat-soluble and, therefore, can be stored in the body and slowly released,
potentially affecting drug tests for several days after CBD use. Also be aware
that even regular hemp oil and hemp seed can
contain small amounts of THC and, at a high enough dose, result in a positive
drug test.
·
Some branches of the U.S. military, including the Army, Air
Force and Coast Guard forbid the use of any hemp-based
products, including hemp oil, hemp seeds, and foods that contain
hemp, such as yogurt containing hemp seeds.
Interestingly, the World Anti-Doping Agency, which helps to oversee drug
policies in sports, announced in 2018
that cannabidiol is no longer prohibited by the organization, but cautioned
that "cannabidiol extracted from cannabis plants may also contain
varying concentrations of THC, which remains a prohibited substance."
(Note: Policies on hemp and CBD consumption in professional sports
organizations still vary based on the organization).
·
The American Academy of Pediatrics has not endorsed Cannabis and
cannabinoid use because of concerns about brain development (National Cancer Institute 2017).
·
In dogs, giving 2 mg per kilogram of bodyweight, twice daily.
(e.g., for a 20 lb dog: 18 mg of CBD in the morning and again at night) for a
month was found to cause an increase in levels of the liver enzyme alkaline
phosphatase, and the researchers recommended monitoring liver enzymes
in dogs receiving CBD until long-term safety studies are conducted (Gamble, Front Vet Sci 2018).
Information on this site
is provided for informational purposes only. It is not an endorsement of any
product nor is it meant to substitute for the advice provided by physicians or
other healthcare professionals. The information contained herein should not be
used for diagnosing or treating a health problem or disease. Consumers should
inform their healthcare providers of the dietary supplements they take.
Latest Clinical Research Updates for CBD & Hemp Extract
Supplements
9/18/2021
A study tested whether
moderate doses of CBD could reduce the pain of hand osteoarthritis or psoriatic
arthritis. See what the study found in the Pain section of our CBD Oil and Hemp
Extracts Review.
8/17/2021
Healthcare workers were
given daily CBD to see if it reduced symptoms of emotional exhaustion and
burnout. Did it work and was it safe? See the findings in the What It Does section of our CBD Oil &
Hemp Extracts Review.
6/19/2021
Be aware that if you
consume caffeine, very high doses of CBD may increase caffeine levels in the
body and, potentially, caffeine side effects, according to a new study. Get the
details about this and other interactions in the Concerns and Cautions section of our CBD
& Hemp Extracts Review. Also see our Top Picks among CBD oils and hemp extracts.
5/04/2021
Can CBD reduce pain
severity or help people better tolerate pain? See what a recent study found in
the What It Does section of our CBD &
Hemp Extract Supplements, Lotions, and Balms Review.
4/17/2021
Does taking CBD help in
treating acute low back pain? See what a recent study found in the What It Does section of our CBD &
Hemp Extract Supplements, Lotions, and Balms Review. Also see our Top Picks among
CBD products.

















