page 32 > Sleep Disorders
Towards understanding the neural origins of hibernation
https://journals.biologists.com/jeb/article/225/1/jeb229542/273864/Towards-understanding-the-neural-origins-of Hibernators thrive under harsh environmental conditions instead of initiating canonical behavioral and physiological responses to promote survival. Although the physiological changes that occur during hibernation have been comprehensively researched, the role of the nervous system in this process remains relatively underexplored. In this Review, we adopt the perspective that the nervous system plays an active, essential role in facilitating and supporting hibernation. Accumulating evidence strongly suggests that the hypothalamus enters a quiescent state in which powerful drives to thermoregulate, eat and drink are suppressed. Similarly, cardiovascular and pulmonary reflexes originating in the brainstem are altered to permit the profoundly slow heart and breathing rates observed during torpor. The mechanisms underlying these changes to the hypothalamus and brainstem are not currently known, but several neuromodulatory systems have been implicated in the induction and maintenance of hibernation. The intersection of these findings with modern neuroscience approaches, such as optogenetics and in vivo calcium imaging, has opened several exciting avenues for hibernation research. Introduction Why study the neurophysiology of hibernation?Life depends upon a certain degree of stability to flourish. Cellular health and organismal survival require the powerful defense of set points, or target values, for parameters such as temperature, oxygen and osmolality. To maintain a consistent internal environment, animals must constantly coordinate numerous physiological responses to environmental changes in a process known as homeostasis. Among species living in harsh climates, these responses can be complex. When faced with cues about the impending winter, some animals will enter the unique state of hibernation (Andrews, 2019; Mohr et al., 2020). A hibernating lifestyle enables survival in the absence of warmth or resources for several months. Studies from the past two centuries have revealed essential insights into the function of individual organ systems during hibernation (Mann, 1916; Rasmussen, 1916). The relationships between organ systems in hibernation, however, remain underexplored. In particular, the field lacks consensus on a working model for how this extraordinary state is initiated and enforced. In this Review, we focus on the central nervous system (CNS) – particularly the hypothalamus and brainstem – as a potential master regulator of hibernation. The CNS exerts powerful influence over the endocrine, cardiovascular, pulmonary and peripheral nervous systems. We propose that it is this exquisite control that affords hibernators resilience in the most harrowing of conditions. Emerging evidence suggests that hibernation is only a variation on the theme of homeostasis, and that hibernators largely rely on the basic biological machinery present in all vertebrates. In studying the neural control of this extreme state, we aim to reveal basic truths about how organ systems are coordinated to achieve stability in an ever-changing world. What is hibernation?Hibernation is a physiological state defined by prolonged (>24 h) bouts of torpor punctuated with arousals (Fig. 1). Torpor bouts are periods of profound energy conservation characterized by a decrease in core body temperature towards ambient levels. Hypothermia in torpor is accompanied by decreases in heart, respiratory and metabolic rates, and a drop in blood pressure. The torpid animal assumes a fetal position from which it rarely moves, sometimes for weeks. Torpor is interrupted periodically with interbout arousals (IBAs). An IBA is a transient state of activity in which the animal will recover active-like physiological parameters such as body temperature, heart rate and blood pressure. 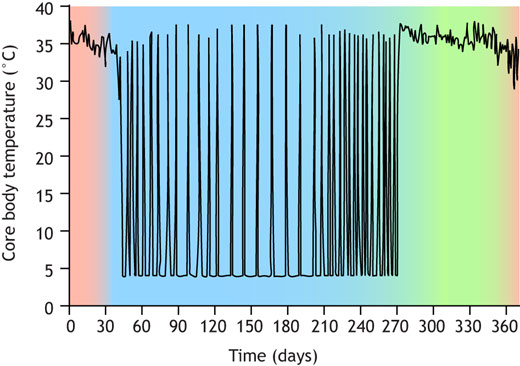 Seasonal changes in body temperature in thirteen-lined ground squirrels. During the summer (green), thirteen-lined ground squirrels are active (core body temperature, Tb=37°C). After passing through a period of heterothermy (red), squirrels hibernate all winter (blue). Within hibernation, squirrels oscillate between torpor (Tb=4°C) and interbout arousal (IBA) (Tb=37°C). The purpose of IBA is not well understood, but it is thought to be important for ‘housekeeping’ metabolic functions (Andrews, 2019). Many species of ground squirrel will urinate and sleep during the ∼24 h they spend in IBA, presumably clearing forms of waste that have accumulated during torpor (Jani et al., 2012; Passmore et al., 1975; Walker et al., 1977; D'Alessandro et al., 2017). Although animals in IBA are mobile and have gone without resources sometimes for months, they remain in their burrows. Ground squirrels in IBA will drink and eat negligible amounts when offered water or food (Feng et al., 2019; Healy et al., 2011). The prioritization of certain behaviors and suppression of others serves as a window into the neuronal mechanisms underlying hibernation. Sleep, urination, eating and drinking are coordinated by the hypothalamus, which integrates interoceptive cues to prioritize behaviors that restore homeostasis. What is the brain's role in hibernation?The hypothalamus and brainstem play an essential role in coordinating behavioral and physiological responses to internal and external stimuli (Bjursten et al., 1976; Mettler et al., 1935) (Fig. 2). For example, specialized neurons in the arcuate nucleus of the hypothalamus (ARC) are responsible for monitoring energy balance (Augustine et al., 2020). When activated, these neurons initiate feeding behavior. Similar processes are known to occur in different hypothalamic regions for fluid homeostasis and thermoregulation (Zimmerman et al., 2017; Angilletta et al., 2019). These circuits do not act alone: instead, they inform each other, allowing for the prioritization of one drive over another. Brainstem nuclei perform a similar function to the hypothalamus, sensing and responding to interoceptive cues by triggering autonomic reflexes, which maintain set points for blood pressure, breathing rate and other organ functions.
Seasonal changes in body temperature in thirteen-lined ground squirrels. During the summer (green), thirteen-lined ground squirrels are active (core body temperature, Tb=37°C). After passing through a period of heterothermy (red), squirrels hibernate all winter (blue). Within hibernation, squirrels oscillate between torpor (Tb=4°C) and interbout arousal (IBA) (Tb=37°C). The purpose of IBA is not well understood, but it is thought to be important for ‘housekeeping’ metabolic functions (Andrews, 2019). Many species of ground squirrel will urinate and sleep during the ∼24 h they spend in IBA, presumably clearing forms of waste that have accumulated during torpor (Jani et al., 2012; Passmore et al., 1975; Walker et al., 1977; D'Alessandro et al., 2017). Although animals in IBA are mobile and have gone without resources sometimes for months, they remain in their burrows. Ground squirrels in IBA will drink and eat negligible amounts when offered water or food (Feng et al., 2019; Healy et al., 2011). The prioritization of certain behaviors and suppression of others serves as a window into the neuronal mechanisms underlying hibernation. Sleep, urination, eating and drinking are coordinated by the hypothalamus, which integrates interoceptive cues to prioritize behaviors that restore homeostasis. What is the brain's role in hibernation?The hypothalamus and brainstem play an essential role in coordinating behavioral and physiological responses to internal and external stimuli (Bjursten et al., 1976; Mettler et al., 1935) (Fig. 2). For example, specialized neurons in the arcuate nucleus of the hypothalamus (ARC) are responsible for monitoring energy balance (Augustine et al., 2020). When activated, these neurons initiate feeding behavior. Similar processes are known to occur in different hypothalamic regions for fluid homeostasis and thermoregulation (Zimmerman et al., 2017; Angilletta et al., 2019). These circuits do not act alone: instead, they inform each other, allowing for the prioritization of one drive over another. Brainstem nuclei perform a similar function to the hypothalamus, sensing and responding to interoceptive cues by triggering autonomic reflexes, which maintain set points for blood pressure, breathing rate and other organ functions. 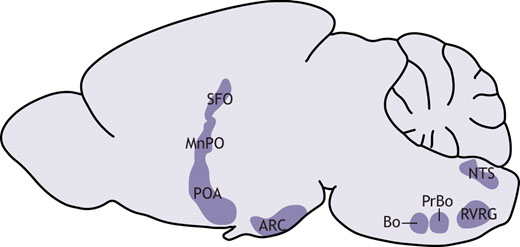 Brain regions involved in homeostasis that may support hibernation. The subfornical organ (SFO) and median preoptic nucleus (MnPO) regulate fluid homeostasis. The preoptic area (POA) is a key player in the neural control of thermoregulation. Neurons in the arcuate nucleus (ARC) control feeding behavior. Nucleus of the solitary tract (NTS) neurons are implicated in the baroreceptor reflex. The Bötzinger (Bo) and pre-Bötzinger complexes (PrBo) and rostral division of the ventral respiratory group (RVRG) are involved in respiration. All of the behavioral and physiological functions mentioned above, as well as others, are altered during hibernation. We propose that these changes are not a result of hibernation, but instead are essential for its induction. Rather than mounting responses to cold exposure and resource deprivation, hibernators suppress powerful drives at the neuronal level. Electroencephalogram studies in hibernators raised questions about neural activity in hibernation over 60 years ago (Strumwasser, 1958; Andersen et al., 1960). The field has since made great progress towards answering these questions, and with the advent of new tools for neural measurement and manipulation, we are surely on the precipice of a deeper understanding of the neurobiology of hibernation. Hypothalamic control of homeostasisThe initiation and maintenance of hibernation require the precise coordination of hypothalamic drives. Hunger, for example, is a powerful motivator that can supersede other important survival signals like inflammatory pain (Alhadeff et al., 2018). It is probable that in species relying solely on body fat for energy during hibernation, the sensation of hunger would be counterproductive. This rationale may be extended to other homeostatic processes, including thirst and thermogenesis. Especially in IBA, animals must suppress any motivation that would otherwise lead them to terminate hibernation prematurely. Consistent with this notion, bulk mRNA sequencing of the hypothalamus across the hibernation cycle highlighted numerous transcriptional changes that may impact neuronal function, protein turnover and specific homeostatic processes (Schwartz et al., 2013). Although brainstem centers are also reported to participate in hunger (Blouet and Schwartz, 2012), thirst (Davern, 2014) and thermoregulation (Morrison and Nakamura, 2011), little is known about how these processes are affected during hibernation. For this reason, we focus in this section on the hypothalamus. HungerHibernators can be categorized as either facultative or obligate. Facultative hibernators enter hibernation in response to environmental cues, such as temperature and food availability, whereas obligate hibernators will do so regardless (Mohr et al., 2020). Obligate hibernators can survive for 5–8 months without food and water, and are the most robust form of hibernator. They adhere to a circannual rhythm in feeding behavior, oscillating between a high-fat appetite and anorexia. In summer, a diet rich in polyunsaturated fatty acids promotes the enormous accumulation of white adipose tissue. Weeks prior to hibernation, however, animals reduce food intake in a period of anorexia that persists for the duration of hibernation, including IBA (Healy et al., 2011; Torke and Twente, 1977; Schwartz et al., 2015). In the neuroendocrine circuit for hunger, reduced food intake will induce the production of ghrelin in the stomach. High circulating ghrelin levels signal hunger to agouti-related peptide (Agrp+) neurons in the arcuate nucleus of the hypothalamus, which are responsible for the coordination of feeding behavior (Augustine et al., 2020). Consequent cues about food intake will rapidly suppress the activity of Agrp+ neurons, as will the production of short- and long-term satiety hormones, including insulin and leptin. A barrage of cues is involved in the neural suppression of feeding behavior; olfactory, gustatory, oropharyngeal and gastrointestinal sensations all contribute, as do endocrine signals. Any combination may be involved in the prolonged anorexia observed in obligate hibernators. One possibility is that ghrelin has a diminished ability to stimulate Agrp+ neurons during hibernation. In golden-mantled ground squirrels, plasma ghrelin levels remain relatively consistent across physiological states (Healy et al., 2010). However, whereas intraperitoneal treatment with ghrelin strongly potentiates food intake in the summer, torpid squirrels forced to arouse in the winter exhibit a much smaller response (Healy et al., 2011). This treatment also elevates hypothalamic levels of AMP-related protein kinase (AMPK) and acetyl-coA carboxylase (ACC) in active, but not hibernating squirrels. Hypothalamic AMPK and ACC are upregulated in response to ghrelin in non-hibernators and may be an essential part of its orexigenic effect (Kohno et al., 2008; Lage et al., 2010; Galic et al., 2018). Thus, it is possible that ghrelin does not achieve the same orexigenic effect during hibernation due to decreased stimulation of intracellular signaling pathways. Consistent with this hypothesis, intracerebroventricular administration of an AMPK activator to yellow-bellied marmots in IBA results in a robust increase in feeding behavior, indicating that activation of hypothalamic AMPK is sufficient to restore hunger (Florant et al., 2010). How might the effects of hunger cues change with season? One potential mechanism involves tanycytes, which are a subset of radial glial cells that help to maintain the blood–hypothalamus barrier. Among other regions, tanycytes line the third ventricle of the brain, where they can regulate the passage of hormones, including ghrelin, from the circulation into the brain to regulate the activity of Agrp+ neurons in the arcuate nucleus (Bolborea et al., 2020). Tanycytic function is influenced by seasonal changes and metabolic status, making these cells prime candidates for investigating the neural mechanism of seasonal anorexia in hibernators (Langlet et al., 2013; Lewis and Ebling, 2017). For example, in non-hibernators, tanycytes have been shown to modulate the blood–hypothalamus barrier in response to fasting (Langlet et al., 2013), which could influence the ability of hormones like ghrelin or leptin to reach Agrp+ neurons. Tanycyte protein expression and morphology are seasonally regulated in arctic ground squirrels and Siberian hamsters (Barrett et al., 2006; Frare and Drew, 2021; Herwig et al., 2009; Nilaweera et al., 2011), supporting a role for tanycytes in hibernation anorexia. Another, non-mutually exclusive possibility is that hunger hormone receptors are differentially expressed across the hibernation cycle. For example, hypothalamic expression of the leptin receptor is upregulated in the period of reduced appetite preceding hibernation (Schwartz et al., 2015; Mohr et al., 2020). The question of whether these molecular changes are necessary and sufficient to maintain hibernation anorexia remains to be answered. Thirst Whereas most mammals cannot last for more than a few days in a state of dehydration, hibernators routinely abstain from drinking water for up to 8 months. When offered water during IBA, thirteen-lined ground squirrels demonstrate a near-complete aversion, revealing that thirst is powerfully inhibited during hibernation (Feng et al., 2019). Water ingestion is coordinated by an endocrine circuit involving the central nervous and renal systems. Dehydration results in elevated blood osmolality, reduced renal perfusion or both. The kidneys' juxtaglomerular apparatus responds by activating the renin–angiotensin–aldosterone system, increasing circulating levels of angiotensin II and aldosterone (Boron and Boulpaep, 2016). These hormones activate neurons in subcortical brain regions that sense dehydration and coordinate a drinking and water-conserving response. In particular, angiotensin II and high osmolality are directly sensed by neurons in the lamina terminalis, a collection of hypothalamic and subcortical brain regions that include the subfornical organ and median preoptic nucleus (Fig. 2). Activation of lamina terminalis neurons promotes water drinking behavior and the release of vasopressin from the posterior pituitary gland (Augustine et al., 2018; Zimmerman et al., 2016). Hibernators may have multiple strategies for preventing water loss and inhibiting thirst during the winter. Although long-term water deprivation usually results in elevated blood electrolyte concentration, torpid thirteen-lined ground squirrels exhibit a significant drop in blood osmolality (Feng et al., 2019; Zimny et al., 1984). A similar phenomenon has been reported in other hibernators, including marmots and prairie dogs (Zatzman and South, 1981; Hamilton and Pfeiffer, 1977). Such a reduction in blood osmolality would be expected to serve as a thirst-suppressing signal to both the kidney and the brain. Plasma renin activity, aldosterone and vasopressin levels are low during torpor, implying that neither the juxtaglomerular apparatus nor thirst neurons are activated (Feng et al., 2019; Kastner et al., 1978). In other words, hypoosmolar blood may inhibit thirst in torpor, but this remains to be experimentally tested. Blood osmolality recovers to active-like levels during IBA, yet unlike active animals, which drink water at some frequency, IBA squirrels abstain from drinking, suggesting that the link between dehydration and thirst is broken in hibernation (Feng et al., 2019). Plasma renin activity and aldosterone levels begin to rise towards the end of the torpor bout, indicating that the kidneys remain sensitive to the squirrel's dehydrated state, albeit less so than in active animals (Kastner et al., 1978). If this is the case, angiotensin II levels would be expected to rise along with renin and aldosterone levels, which remains to be shown. Because IBA squirrels do not drink water despite the sharp increase in osmolarity and renal hormones during the torpor–IBA transition, it is possible that the suppression of thirst is enforced by lamina terminalis neurons. Acute hyperosmotic stimuli, such as the intraperitoneal injection of sodium chloride or mannitol, partially restore thirst, suggesting that the suppression is reversible and the thirst neural circuit can detect and respond to large changes in osmolarity during hibernation (Feng et al., 2019). Additionally, it is important to note that squirrels preparing for hibernation – in ‘prehibernation torpor’ – also exhibit reduced thirst, but still drink significantly more than IBA squirrels. Furthermore, squirrels in prehibernation torpor have lower blood osmolality than IBA squirrels, which would be expected to inhibit thirst (Feng et al., 2019). This suggests that both seasonality and hibernation status contribute to drinking behavior in the thirteen-lined ground squirrel. The exact mechanism by which thirst inhibition emerges remains to be explored, but it is tempting to speculate that lamina terminalis neurons could play a pivotal role in this process. Thermogenesis Hibernators are heterothermic during the autumn and winter, meaning that their body temperature is highly variable, and some species achieve a body temperature that is not much higher than ambient levels. By contrast, non-hibernating mammals have a constant body temperature set point, deviations from which engage physiological and behavioral responses. A decrease in body temperature triggers shivering and non-shivering brown adipose tissue thermogenesis, vasoconstriction and heat-seeking behavior (Boron and Boulpaep, 2016). Temperature changes are jointly sensed by the peripheral and central nervous systems. Specialized somatosensory neurons from dorsal root ganglia (DRG) and trigeminal ganglia innervate the integumentary system, where they directly sense environmental temperature. Somatosensory neurons that express the TRPM8 ion channel are activated by cooling, whereas those that express TRPV1 sense heat (Bautista et al., 2007; Caterina et al., 1997). Heat- and cold-sensitive somatosensory neurons connect to a multisynaptic circuit in the CNS that delivers temperature information to the preoptic area (POA) of the hypothalamus. Separate subsets of POA neurons are dedicated to sensing increases and decreases in brain temperature (Feketa et al., 2020; Tan and Knight, 2018). POA neurons receiving information about the external and internal temperatures will then trigger the thermoregulatory responses described above. Because hibernators experience decreases in both body and brain temperature, they must suppress the drive for thermogenesis in both of these regions. In thirteen-lined ground squirrels, thermoregulatory responses are almost completely abolished during torpor. Although brown adipose tissue (BAT) and brain cortex temperature are both slightly higher than environmental temperature, the greatest difference is only around 1°C, reflecting a near-complete suppression of the cold defense response (Laursen et al., 2015). Interestingly, cortex temperature is slightly, but significantly, higher than BAT temperature (Laursen et al., 2015), supporting the notion that brain and body temperature set points may not be defended by the same mechanism. It is possible that thermoregulatory suppression comes in part from impaired thermosensation arising from the peripheral nervous system (PNS). Thirteen-lined ground squirrels and Syrian hamsters exhibit remarkable tolerance for the sensation of cold when compared with mice (Matos-Cruz et al., 2017). This cold tolerance is observed not only at the behavioral, but also the cellular level: squirrel DRG neurons are less sensitive to colder temperatures than mouse DRG neurons (Matos-Cruz et al., 2017). Furthermore, both squirrels and hamsters express a cold-insensitive ortholog of TRPM8 that likely partially contributes to both cellular and behavioral cold tolerance (Matos-Cruz et al., 2017) (Fig. 3).
Brain regions involved in homeostasis that may support hibernation. The subfornical organ (SFO) and median preoptic nucleus (MnPO) regulate fluid homeostasis. The preoptic area (POA) is a key player in the neural control of thermoregulation. Neurons in the arcuate nucleus (ARC) control feeding behavior. Nucleus of the solitary tract (NTS) neurons are implicated in the baroreceptor reflex. The Bötzinger (Bo) and pre-Bötzinger complexes (PrBo) and rostral division of the ventral respiratory group (RVRG) are involved in respiration. All of the behavioral and physiological functions mentioned above, as well as others, are altered during hibernation. We propose that these changes are not a result of hibernation, but instead are essential for its induction. Rather than mounting responses to cold exposure and resource deprivation, hibernators suppress powerful drives at the neuronal level. Electroencephalogram studies in hibernators raised questions about neural activity in hibernation over 60 years ago (Strumwasser, 1958; Andersen et al., 1960). The field has since made great progress towards answering these questions, and with the advent of new tools for neural measurement and manipulation, we are surely on the precipice of a deeper understanding of the neurobiology of hibernation. Hypothalamic control of homeostasisThe initiation and maintenance of hibernation require the precise coordination of hypothalamic drives. Hunger, for example, is a powerful motivator that can supersede other important survival signals like inflammatory pain (Alhadeff et al., 2018). It is probable that in species relying solely on body fat for energy during hibernation, the sensation of hunger would be counterproductive. This rationale may be extended to other homeostatic processes, including thirst and thermogenesis. Especially in IBA, animals must suppress any motivation that would otherwise lead them to terminate hibernation prematurely. Consistent with this notion, bulk mRNA sequencing of the hypothalamus across the hibernation cycle highlighted numerous transcriptional changes that may impact neuronal function, protein turnover and specific homeostatic processes (Schwartz et al., 2013). Although brainstem centers are also reported to participate in hunger (Blouet and Schwartz, 2012), thirst (Davern, 2014) and thermoregulation (Morrison and Nakamura, 2011), little is known about how these processes are affected during hibernation. For this reason, we focus in this section on the hypothalamus. HungerHibernators can be categorized as either facultative or obligate. Facultative hibernators enter hibernation in response to environmental cues, such as temperature and food availability, whereas obligate hibernators will do so regardless (Mohr et al., 2020). Obligate hibernators can survive for 5–8 months without food and water, and are the most robust form of hibernator. They adhere to a circannual rhythm in feeding behavior, oscillating between a high-fat appetite and anorexia. In summer, a diet rich in polyunsaturated fatty acids promotes the enormous accumulation of white adipose tissue. Weeks prior to hibernation, however, animals reduce food intake in a period of anorexia that persists for the duration of hibernation, including IBA (Healy et al., 2011; Torke and Twente, 1977; Schwartz et al., 2015). In the neuroendocrine circuit for hunger, reduced food intake will induce the production of ghrelin in the stomach. High circulating ghrelin levels signal hunger to agouti-related peptide (Agrp+) neurons in the arcuate nucleus of the hypothalamus, which are responsible for the coordination of feeding behavior (Augustine et al., 2020). Consequent cues about food intake will rapidly suppress the activity of Agrp+ neurons, as will the production of short- and long-term satiety hormones, including insulin and leptin. A barrage of cues is involved in the neural suppression of feeding behavior; olfactory, gustatory, oropharyngeal and gastrointestinal sensations all contribute, as do endocrine signals. Any combination may be involved in the prolonged anorexia observed in obligate hibernators. One possibility is that ghrelin has a diminished ability to stimulate Agrp+ neurons during hibernation. In golden-mantled ground squirrels, plasma ghrelin levels remain relatively consistent across physiological states (Healy et al., 2010). However, whereas intraperitoneal treatment with ghrelin strongly potentiates food intake in the summer, torpid squirrels forced to arouse in the winter exhibit a much smaller response (Healy et al., 2011). This treatment also elevates hypothalamic levels of AMP-related protein kinase (AMPK) and acetyl-coA carboxylase (ACC) in active, but not hibernating squirrels. Hypothalamic AMPK and ACC are upregulated in response to ghrelin in non-hibernators and may be an essential part of its orexigenic effect (Kohno et al., 2008; Lage et al., 2010; Galic et al., 2018). Thus, it is possible that ghrelin does not achieve the same orexigenic effect during hibernation due to decreased stimulation of intracellular signaling pathways. Consistent with this hypothesis, intracerebroventricular administration of an AMPK activator to yellow-bellied marmots in IBA results in a robust increase in feeding behavior, indicating that activation of hypothalamic AMPK is sufficient to restore hunger (Florant et al., 2010). How might the effects of hunger cues change with season? One potential mechanism involves tanycytes, which are a subset of radial glial cells that help to maintain the blood–hypothalamus barrier. Among other regions, tanycytes line the third ventricle of the brain, where they can regulate the passage of hormones, including ghrelin, from the circulation into the brain to regulate the activity of Agrp+ neurons in the arcuate nucleus (Bolborea et al., 2020). Tanycytic function is influenced by seasonal changes and metabolic status, making these cells prime candidates for investigating the neural mechanism of seasonal anorexia in hibernators (Langlet et al., 2013; Lewis and Ebling, 2017). For example, in non-hibernators, tanycytes have been shown to modulate the blood–hypothalamus barrier in response to fasting (Langlet et al., 2013), which could influence the ability of hormones like ghrelin or leptin to reach Agrp+ neurons. Tanycyte protein expression and morphology are seasonally regulated in arctic ground squirrels and Siberian hamsters (Barrett et al., 2006; Frare and Drew, 2021; Herwig et al., 2009; Nilaweera et al., 2011), supporting a role for tanycytes in hibernation anorexia. Another, non-mutually exclusive possibility is that hunger hormone receptors are differentially expressed across the hibernation cycle. For example, hypothalamic expression of the leptin receptor is upregulated in the period of reduced appetite preceding hibernation (Schwartz et al., 2015; Mohr et al., 2020). The question of whether these molecular changes are necessary and sufficient to maintain hibernation anorexia remains to be answered. Thirst Whereas most mammals cannot last for more than a few days in a state of dehydration, hibernators routinely abstain from drinking water for up to 8 months. When offered water during IBA, thirteen-lined ground squirrels demonstrate a near-complete aversion, revealing that thirst is powerfully inhibited during hibernation (Feng et al., 2019). Water ingestion is coordinated by an endocrine circuit involving the central nervous and renal systems. Dehydration results in elevated blood osmolality, reduced renal perfusion or both. The kidneys' juxtaglomerular apparatus responds by activating the renin–angiotensin–aldosterone system, increasing circulating levels of angiotensin II and aldosterone (Boron and Boulpaep, 2016). These hormones activate neurons in subcortical brain regions that sense dehydration and coordinate a drinking and water-conserving response. In particular, angiotensin II and high osmolality are directly sensed by neurons in the lamina terminalis, a collection of hypothalamic and subcortical brain regions that include the subfornical organ and median preoptic nucleus (Fig. 2). Activation of lamina terminalis neurons promotes water drinking behavior and the release of vasopressin from the posterior pituitary gland (Augustine et al., 2018; Zimmerman et al., 2016). Hibernators may have multiple strategies for preventing water loss and inhibiting thirst during the winter. Although long-term water deprivation usually results in elevated blood electrolyte concentration, torpid thirteen-lined ground squirrels exhibit a significant drop in blood osmolality (Feng et al., 2019; Zimny et al., 1984). A similar phenomenon has been reported in other hibernators, including marmots and prairie dogs (Zatzman and South, 1981; Hamilton and Pfeiffer, 1977). Such a reduction in blood osmolality would be expected to serve as a thirst-suppressing signal to both the kidney and the brain. Plasma renin activity, aldosterone and vasopressin levels are low during torpor, implying that neither the juxtaglomerular apparatus nor thirst neurons are activated (Feng et al., 2019; Kastner et al., 1978). In other words, hypoosmolar blood may inhibit thirst in torpor, but this remains to be experimentally tested. Blood osmolality recovers to active-like levels during IBA, yet unlike active animals, which drink water at some frequency, IBA squirrels abstain from drinking, suggesting that the link between dehydration and thirst is broken in hibernation (Feng et al., 2019). Plasma renin activity and aldosterone levels begin to rise towards the end of the torpor bout, indicating that the kidneys remain sensitive to the squirrel's dehydrated state, albeit less so than in active animals (Kastner et al., 1978). If this is the case, angiotensin II levels would be expected to rise along with renin and aldosterone levels, which remains to be shown. Because IBA squirrels do not drink water despite the sharp increase in osmolarity and renal hormones during the torpor–IBA transition, it is possible that the suppression of thirst is enforced by lamina terminalis neurons. Acute hyperosmotic stimuli, such as the intraperitoneal injection of sodium chloride or mannitol, partially restore thirst, suggesting that the suppression is reversible and the thirst neural circuit can detect and respond to large changes in osmolarity during hibernation (Feng et al., 2019). Additionally, it is important to note that squirrels preparing for hibernation – in ‘prehibernation torpor’ – also exhibit reduced thirst, but still drink significantly more than IBA squirrels. Furthermore, squirrels in prehibernation torpor have lower blood osmolality than IBA squirrels, which would be expected to inhibit thirst (Feng et al., 2019). This suggests that both seasonality and hibernation status contribute to drinking behavior in the thirteen-lined ground squirrel. The exact mechanism by which thirst inhibition emerges remains to be explored, but it is tempting to speculate that lamina terminalis neurons could play a pivotal role in this process. Thermogenesis Hibernators are heterothermic during the autumn and winter, meaning that their body temperature is highly variable, and some species achieve a body temperature that is not much higher than ambient levels. By contrast, non-hibernating mammals have a constant body temperature set point, deviations from which engage physiological and behavioral responses. A decrease in body temperature triggers shivering and non-shivering brown adipose tissue thermogenesis, vasoconstriction and heat-seeking behavior (Boron and Boulpaep, 2016). Temperature changes are jointly sensed by the peripheral and central nervous systems. Specialized somatosensory neurons from dorsal root ganglia (DRG) and trigeminal ganglia innervate the integumentary system, where they directly sense environmental temperature. Somatosensory neurons that express the TRPM8 ion channel are activated by cooling, whereas those that express TRPV1 sense heat (Bautista et al., 2007; Caterina et al., 1997). Heat- and cold-sensitive somatosensory neurons connect to a multisynaptic circuit in the CNS that delivers temperature information to the preoptic area (POA) of the hypothalamus. Separate subsets of POA neurons are dedicated to sensing increases and decreases in brain temperature (Feketa et al., 2020; Tan and Knight, 2018). POA neurons receiving information about the external and internal temperatures will then trigger the thermoregulatory responses described above. Because hibernators experience decreases in both body and brain temperature, they must suppress the drive for thermogenesis in both of these regions. In thirteen-lined ground squirrels, thermoregulatory responses are almost completely abolished during torpor. Although brown adipose tissue (BAT) and brain cortex temperature are both slightly higher than environmental temperature, the greatest difference is only around 1°C, reflecting a near-complete suppression of the cold defense response (Laursen et al., 2015). Interestingly, cortex temperature is slightly, but significantly, higher than BAT temperature (Laursen et al., 2015), supporting the notion that brain and body temperature set points may not be defended by the same mechanism. It is possible that thermoregulatory suppression comes in part from impaired thermosensation arising from the peripheral nervous system (PNS). Thirteen-lined ground squirrels and Syrian hamsters exhibit remarkable tolerance for the sensation of cold when compared with mice (Matos-Cruz et al., 2017). This cold tolerance is observed not only at the behavioral, but also the cellular level: squirrel DRG neurons are less sensitive to colder temperatures than mouse DRG neurons (Matos-Cruz et al., 2017). Furthermore, both squirrels and hamsters express a cold-insensitive ortholog of TRPM8 that likely partially contributes to both cellular and behavioral cold tolerance (Matos-Cruz et al., 2017) (Fig. 3). 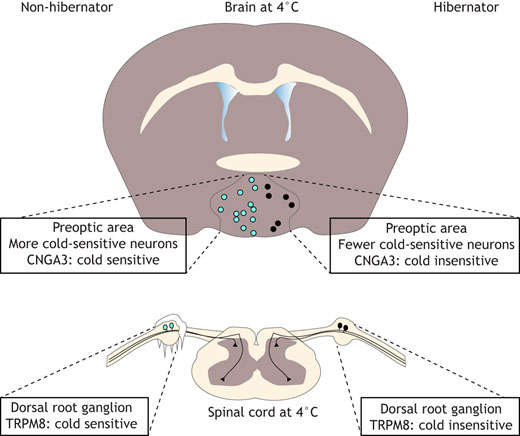 Thermoregulatory responses to cold. Hibernators express cold-insensitive orthologs of TRPM8 in dorsal root ganglion (DRG) neurons and CNGA3 in preoptic area (POA) hypothalamic neurons. Additionally, there are fewer cold-sensitive neurons in the POA of hibernators versus non-hibernators. Thus, DRG and POA neurons from hibernators cannot respond as strongly to cold exposure. Neuronal response to cold is represented in light blue; insensitivity to cold in black. In non-hibernators, hypothalamic cooling is sufficient to trigger a thermoregulatory response (Satinoff, 1964). By contrast, hibernators exhibit extremely low brain and body temperatures in torpor, suggesting that thermoregulatory suppression is supported by the CNS in addition to the PNS mechanism described above. Research in thirteen-lined ground squirrels has provided some insight into the role of the CNS in this process. Compared with mice, squirrels have fewer intrinsically cold-sensitive POA neurons (Feketa et al., 2020). Although the molecular basis of cold sensitivity in POA neurons remains unclear, it was suggested that it is partially mediated by CNGA3, a non-selective excitatory cyclic nucleotide gated ion channel present in POA neurons of mice and squirrels. The activity of mouse CNGA3 is robustly potentiated by cooling; however, no such potentiation is observed in the squirrel ortholog (Feketa et al., 2020). Thus, CNS cold sensors permit thermoregulatory suppression during torpor in a manner analogous to the PNS mechanism (Fig. 3). It is worth noting that in rats, which do not hibernate, silencing output from POA neurons can trigger a unique state known as thermoregulatory inversion, in which cold exposure facilitates a decrease in body temperature instead of an increase (Tupone et al., 2017). As such, thermoregulatory inversion appears to mimic some features of hibernation; however, the extent to which these phenomena overlap remains to be determined. In summary, it is likely that hibernators possess an impaired ability to sense the decreases in brain and body temperature that would normally trigger a thermogenic response, thereby enabling heterothermy. Brainstem control of homeostasis Brainstem nuclei promote other forms of homeostasis by coordinating autonomic responses. These include robust cardiovascular and pulmonary reflexes, which must be quelled during the hibernation season to prevent premature emergence. During entry into torpor, for example, blood pressure and heart rate drop concomitantly (Chatfield and Lyman, 1950; Lyman and O'Brien, 1961; Horwitz et al., 2013). This phenomenon is indicative of dramatic changes to autonomic processes designed to compensate for blood pressure fluctuations with adjustments to heart rate (Zeng et al., 2018). Significant insights have been made into the molecular mechanisms protecting hibernators from ischemia, reperfusion and hypoxia (Dave et al., 2012), but considerably less is known about how the nervous system permits these conditions in the first place. Cardiovascular function The autonomic nervous system (ANS) regulates blood pressure and heart rate to maintain precise control over cardiovascular function (Boron and Boulpaep, 2016). In the case of a drop in blood pressure, mechanosensitive baroreceptor neurons sense a decrease in arterial stretch, resulting in a lower firing frequency. These neurons project via the glossopharyngeal nerve to the brainstem. A reduction in firing rate indirectly engages an increase in sympathetic tone. Sympathetic efferents deliver norepinephrine to the cardiovascular system, promoting vasoconstriction, tachycardia and increased contractility of cardiac muscle, and resulting in a near-instantaneous elevation in blood pressure. Parasympathetic efferents, which rely on acetylcholine to slow cardiovascular function, are concurrently inhibited (Boron and Boulpaep, 2016). In stark contrast to non-hibernators, animals entering torpor exhibit a simultaneous, precipitous drop in heart rate and blood pressure that precedes the decrease in body temperature (Chatfield and Lyman, 1950; Horwitz et al., 2013; Lyman and O'Brien, 1961). In Gould's long-eared bats, heart rate and metabolic rate are positively correlated in daily torpor, but in a manner that is distinct from their euthermic conspecifics (Currie et al., 2014). This finding supports the notion that the cardiovascular system behaves differently during periods of heterothermy. Torpor continues for days or weeks with remarkably subdued cardiac function, indicating that the ANS is permissive of this state. The baroreceptor reflex is almost completely suppressed in torpid Syrian hamsters and remains so until late into the ensuing IBA (Horwitz et al., 2013). However, during entry into torpor, the sensitivity of the baroreceptor reflex remains high, suggesting that it plays an active role in the initiation process, perhaps by adjusting the set point for BP (Horwitz et al., 2013). The mechanisms underlying changes to the baroreceptor reflex in hibernation are not known. One possibility is the alteration of baroreceptor sensitivity, e.g. via changes in the function of Piezo1 and Piezo2, mechanically gated ion channels that can detect arterial stretch (Huo et al., 2021; Min et al., 2019; Zeng et al., 2018). Another is the alteration in baroreceptor signaling. Baroreceptor neurons in the carotid artery synapse onto ‘second-order’ neurons residing in the nucleus of the solitary tract (NTS) (Sekizawa et al., 2009) (Fig. 2). One group reported that NTS neurons exhibited different electrophysiological properties in euthermic versus torpid Syrian hamsters (Sekizawa et al., 2013). Although neurons from both states had similar resting membrane potentials, NMDA-induced currents were smaller in torpid neurons at membrane voltages lower than −30 mV. Furthermore, NMDA receptor-independent inputs from first-order baroreceptor neurons from hibernating hamsters resulted in excitatory postsynaptic currents (EPSCs) that decayed more quickly when compared with EPSCs in neurons from euthermic hamsters. These changes suggest a difference in signaling between baroreceptors and second-order neurons in NTS across the hibernation cycle. The baroreceptor reflex engages the parasympathetic (PSNS) and sympathetic nervous (SNS) systems to maintain bidirectional control over heart rate, and there is pharmacological evidence that both systems may be greatly altered during hibernation. PSNS blockade with veratramine, atropine or via vagotomy does not trigger the expected increase in heart rate in torpid thirteen-lined tree squirrels, indicating that the PSNS is almost completely suppressed (Lyman and O'Brien, 1963; Milsom et al., 1999). Supporting this, the SNS agonist norepinephrine elicits hypertension and compensatory bradycardia in active ground squirrels, whereas the same treatment during torpor paradoxically triggers tachycardia (Lyman and O'Brien, 1961). Since the PSNS is responsible for slowing the heart rate, this result serves as indirect evidence of its suppression while the SNS remains partly intact. However, although torpid ground squirrels are still responsive to SNS antagonists in a β-adrenergic receptor-dependent manner, SNS activity is likely to be greatly suppressed compared with levels in an active animal because heart rate and blood pressure are so low (Milsom et al., 2001). Pulmonary function The respiratory system serves to maintain the appropriate levels of blood oxygenation and blood acidity. Deviations from these set points are sensed by peripheral chemoreceptors, which fire action potentials in response to hypoxia, hypercarbia and low pH (Boron and Boulpaep, 2016). This information is conveyed by several cranial nerves to respiratory neurons in the medulla oblongata, including neurons of the Bötzinger and pre-Bötzinger complexes, and rostral division of the ventral respiratory group (Fig. 2). There, a sensory subpopulation receives input from the peripheral chemoreceptors and projects to premotor and motor populations, which coordinate an appropriate breathing response (Boron and Boulpaep, 2016). Of note, a specialized set of premotor neurons is also involved in the modulation of arousal states in mice (Yackle et al., 2017). Within torpor, hibernators may breathe as infrequently as once per minute, suggesting that the respiratory system is radically altered (McArthur and Milsom, 1991). In golden-mantled ground squirrels, this is in part a passive consequence accompanying the reduction in respiratory drive, since metabolic rate is suppressed and blood pH becomes slightly elevated in several species (Kim and Milsom, 2019), but evidence suggests that the neural control of respiratory rhythm is also actively modulated. Many hibernators exhibit a pattern of episodic breathing characterized by clusters of fast, evenly spaced breaths interspersed with prolonged apneas (McArthur and Milsom, 1991; Milsom et al., 2001). This is in stark contrast to euthermic mammals, in which respiratory rhythm remains constant. Whereas euthermic mammals usually alter breath volume and duration to adjust blood oxygenation and pH, torpid golden-mantled ground squirrels instead adjust breath frequency and apnea duration (Milsom et al., 2001). Neither the physiological significance nor the neural mechanisms underlying the different breathing patterns are known, but there are some clues. One study revealed that cooling below 4°C restored evenly spaced breathing in torpid golden-mantled ground squirrels (Milsom et al., 1997). The authors posit that the disappearance of apneas and clustered high-frequency breaths is due to the removal of inhibitory and excitatory inputs, respectively, leaving only the basic pattern generated by respiratory neurons. Independently, neither pharmacological blockade of NMDA receptors nor vagotomy eliminated episodic breathing. However, combining both approaches led to evenly spaced breaths and rapid emergence from torpor (Milsom et al., 1997). Thus, it is possible that some combination of sensory input from the vagus nerve and glutamatergic input to respiratory neurons is required for the unusual rhythm observed in torpor. Recent work has revealed that changes in the expression of GABA¬A receptor subunits across the hibernation cycle produce functional differences in respiratory neurons, hinting at the presence of other molecular mechanisms (Hengen et al., 2009, 2011). Neuromodulation in hibernation We have discussed how hibernation is facilitated and reinforced by significant alterations to brainstem and hypothalamus function. The process by which these changes come about is not known, but evidence supports the involvement of several neuromodulators, including adenosine, endorphins, histamine, thyroid hormone, endocannabinoids and glutamate (Beckman et al., 1981; Frare et al., 2021; Jinka et al., 2012, 2011; Mulawa et al., 2018; Sallmen et al., 1999). By exploring the complex interactions between neuromodulatory systems in hibernation, the field of hibernation research may approach its major goal of determining whether it is possible to induce synthetic torpor in non-hibernators. Adenosine Adenosine is known to be important for the neural control of sleep, and recent work suggests that it may have similar significance for the induction and maintenance of hibernation (Silvani et al., 2018). As an essential component of the ATP pathway, this molecule may serve as a readout of metabolic state (Tupone et al., 2013). In the context of sleep, adenosine accumulates in the basal forebrain throughout the day, activating neurons in the ventrolateral preoptic area of the hypothalamus (VLPO), which in turn inhibit subcortical neurotransmission associated with waking behavior (Drew et al., 2017; Saper et al., 2010). Although it is not known whether a similar accumulation of adenosine occurs circannually in obligate hibernators, there is evidence that this neuromodulator is involved in torpor entry and maintenance. Intracerebroventricular administration of an adenosine A1 receptor (A1AR) antagonist prevents torpor entry in AGS and induces emergence from hibernation in Syrian hamsters (Jinka et al., 2011; Tamura et al., 2005). Furthermore, in AGS, the A1AR agonist N6-cyclohexyladenosine (CHA) promotes torpor entry (Jinka et al., 2011). The effect is only apparent in the winter, suggesting that other seasonal changes are required to sensitize the brain to adenosine. In the rat, which does not hibernate, intracerebroventricular CHA delivery in combination with cooling of environmental temperature significantly lowers body temperature, heart rate and electroencephalogram amplitude, inhibits thermogenesis and can also induce a state of thermoregulatory inversion mentioned above (see ‘Thermogenesis’ section; Tupone et al., 2013, 2017). The activation of A1AR+ neurons in the brainstem nucleus of the solitary tract (NTS) is sufficient to induce this torpor-like state. This treatment increases blood pressure and parasympathetic tone (Tupone et al., 2013), suggesting that it does not recapitulate every feature of hibernation (see ‘Cardiovascular function’ section). Nonetheless, this work highlights A1AR+ NTS neurons as intriguing candidates to study in hibernators. It is also important to note that other studies in non-hibernators have found that CHA-induced hypothermia may occur independently of central A1AR activation (Province et al., 2020). This discrepancy underscores the need for further investigation of adenosinergic signaling in hypothermic states, including hibernation. Endorphins Evidence suggests that hibernation is promoted by the action of endogenous opioids, known as endorphins. These neuropeptides are perhaps most famous for their role in masking pain following strenuous exercise, but they have been implicated in a wide array of physiological processes. Their neuromodulatory effect is enacted through opioid receptors distributed widely throughout the brain (Bourhim et al., 1997). The activation of mu, kappa and delta opioid receptors have been associated with changes in appetite, thirst, body temperature and physical activity similar to those seen across the hibernation cycle (Bodnar, 2020). High concentrations of endorphins have been detected in the brains of hibernators during the winter (Cui et al., 1996; Kramarova et al., 1983). Additionally, the administration of opioid receptor antagonists, including naloxone, is known to interfere with hibernation maintenance in several species (Beckman and Llados-Eckman, 1985; Kromer, 1980; Margules et al., 1979; Tamura et al., 2005). The activation of opioid receptors is known to elicit effects often associated with hibernation, such as analgesia, hypothermia, bradycardia and slow breathing rate (Ban et al., 2020; Bodnar, 2020; Cintron-Colon et al., 2019). It is thus tempting to speculate that endorphinergic signaling is required for hibernation in several species. However, the field has yet to establish a connection between the molecular actions of endorphins with the neurophysiological changes that occur in hibernation. Conclusion In the past two centuries, researchers have made great progress towards understanding the phenomenon of hibernation. Accumulated evidence strongly suggests that the hypothalamus and brainstem play an essential role in the process by adjusting homeostatic set points, and this knowledge highlights several exciting research directions. Modern neuroscience tools will allow us to clearly define the neural underpinnings of the hibernation phenotype, drawing us nearer to unlocking these mechanisms in non-hibernators. Opto- and chemogenetic manipulations, as well as in vivo calcium imaging methods, have been successfully applied in non-standard animal models such as the prairie vole (Amadei et al., 2017; Scribner et al., 2020) and the zebra finch (Picardo et al., 2016; Singh Alvarado et al., 2021). Contemporaneous studies have identified discrete hypothalamic neuronal populations that regulate fasting-induced torpor in mice (Hrvatin et al., 2020) and a hibernation-like state in rats (Takahashi et al., 2020). Going forward, it will be essential to manipulate these discrete neuronal populations, and the other neural circuits mentioned in this Review, using opto- or chemogenetics in true hibernators to determine their necessity and sufficiency in this complex physiological phenomenon. The stage is set for a new era of research into the neurobiology of hibernation. ReferencesAlhadeff, A. L., Su, Z., Hernandez, E., Klima, M. L., Phillips, S. Z., Holland, R. A., Guo, C., Hantman, A. W., De Jonghe, B. C. and Betley, J. N. (2018). A neural circuit for the suppression of pain by a competing need state. Cell 173, 140-152.e15. https://doi.org/10.1016/j.cell.2018.02.057Google ScholarAmadei, E. A., Johnson, Z. V., Jun Kwon, Y., Shpiner, A. C., Saravanan, V., Mays, W. D., Ryan, S. J., Walum, H., Rainnie, D. G., Young, L. J. et al. (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297-301. https://doi.org/10.1038/nature22381Google ScholarAndersen, P., Johansen, K., Krog, J. (1960). Electroencephalogram during arousal from hibernation in the birchmouse. Am. J. Physiol. 199, 535-538. https://doi.org/10.1152/ajplegacy.1960.199.3.535Google ScholarAndrews, M. T. (2019). Molecular interactions underpinning the phenotype of hibernation in mammals. J. Exp. Biol. 222, jeb160606. https://doi.org/10.1242/jeb.160606Google ScholarAngilletta, M. J., Jr, Youngblood, J. P., Neel, L. K. and VandenBrooks, J. M. (2019). The neuroscience of adaptive thermoregulation. Neurosci. Lett. 692, 127-136. https://doi.org/10.1016/j.neulet.2018.10.046Google ScholarAugustine, V., Gokce, S. K., Lee, S., Wang, B., Davidson, T. J., Reimann, F., Gribble, F., Deisseroth, K., Lois, C. and Oka, Y. (2018). Hierarchical neural architecture underlying thirst regulation. Nature 555, 204-209. https://doi.org/10.1038/nature25488Google ScholarAugustine, V., Lee, S. and Oka, Y. (2020). Neural control and modulation of thirst, sodium appetite, and hunger. Cell 180, 25-32. https://doi.org/10.1016/j.cell.2019.11.040Google ScholarBan, E. G., Brassai, A. and Vizi, E. S. (2020). The role of the endogenous neurotransmitters associated with neuropathic pain and in the opioid crisis: the innate pain-relieving system. Brain Res. Bull. 155, 129-136. https://doi.org/10.1016/j.brainresbull.2019.12.001Google ScholarBarrett, P., Ivanova, E., Graham, E. S., Ross, A. W., Wilson, D., Plé, H., Mercer, J. G., Ebling, F. J., Schuhler, S., Dupré, S. M. et al. (2006). Photoperiodic regulation of cellular retinol binding protein, CRBP1 [corrected] and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J. Endocrinol. 191, 687-698. https://doi.org/10.1677/joe.1.06929Google ScholarBautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I., Stucky, C. L., Jordt, S.-E. and Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204-208. https://doi.org/10.1038/nature05910Google ScholarBeckman, A. L. and Llados-Eckman, C. (1985). Antagonism of brain opioid peptide action reduces hibernation bout duration. Brain Res. 328, 201-205. https://doi.org/10.1016/0006-8993(85)91030-3Google ScholarBeckman, A. L., Llados-Eckman, C., Stanton, T. L. and Adler, M. W. (1981). Physical dependence on morphine fails to develop during the hibernating state. Science 212, 1527-1529. https://doi.org/10.1126/science.7233241Google ScholarBjursten, L. M., Norrsell, K. and Norrsell, U. (1976). Behavioural repertory of cats without cerebral cortex from infancy. Exp. Brain Res. 25, 115-130. https://doi.org/10.1007/BF00234897Google ScholarBlouet, C. and Schwartz, G. J. (2012). Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579-587. https://doi.org/10.1016/j.cmet.2012.10.003Google ScholarBodnar, R. J. (2020). Endogenous opiates and behavior: 2017. Peptides 124, 170223. https://doi.org/10.1016/j.peptides.2019.170223Google ScholarBolborea, M., Pollatzek, E., Benford, H., Sotelo-Hitschfeld, T. and Dale, N. (2020). Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc. Natl. Acad. Sci. USA 117, 14473-14481. https://doi.org/10.1073/pnas.1919887117Google ScholarBoron, W. F. and Boulpaep, E. L. (2016). Boron & Boulpaep Concise Medical Physiology. Elsevier Health Sciences.Google ScholarBourhim, N., Kabine, M. and Elkebbaj, M. S. (1997). Characterization of opioid peptides and opioid receptors in the brain of jerboa (Jaculus orientalis), a hibernating rodent. Brain Res. Bull. 44, 615-620. https://doi.org/10.1016/S0361-9230(97)00282-7Google ScholarCaterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816-824. https://doi.org/10.1038/39807Google ScholarChatfield, P. O. and Lyman, C. P. (1950). Circulatory changes during process of arousal in the hibernating hamster. Am. J. Physiol. 163, 566-574. https://doi.org/10.1152/ajplegacy.1950.163.3.566Google ScholarCintron-Colon, R., Johnson, C. W., Montenegro-Burke, J. R., Guijas, C., Faulhaber, L., Sanchez-Alavez, M., Aguirre, C. A., Shankar, K., Singh, M., Galmozzi, A. et al. (2019). Activation of kappa opioid receptor regulates the hypothermic response to calorie restriction and limits body weight loss. Curr. Biol. 29, 4291-4299.e4. https://doi.org/10.1016/j.cub.2019.10.027Google ScholarCui, Y., Lee, T. F. and Wang, L. C. (1996). State-dependent changes of brain endogenous opioids in mammalian hibernation. Brain Res. Bull. 40, 129-133. https://doi.org/10.1016/0361-9230(96)00038-XGoogle ScholarCurrie, S. E., Körtner, G. and Geiser, F. (2014). Heart rate as a predictor of metabolic rate in heterothermic bats. J. Exp. Biol. 217, 1519-1524. https://doi.org/10.1242/jeb.098970Google ScholarDave, K. R., Christian, S. L., Perez-Pinzon, M. A. and Drew, K. L. (2012). Neuroprotection: lessons from hibernators. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 162, 1-9. https://doi.org/10.1016/j.cbpb.2012.01.008Google ScholarDavern, P. J. (2014). A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Front. Physiol. 5, 436. https://doi.org/10.3389/fphys.2014.00436Google ScholarD'Alessandro, A., Nemkov, T., Bogren, L. K., Martin, S. L. and Hansen, K. C. (2017). comfortably numb and back: plasma metabolomics reveals biochemical adaptations in the hibernating 13-lined ground squirrel. J. Proteome Res. 16, 958-969. https://doi.org/10.1021/acs.jproteome.6b00884Google ScholarDrew, K. L., Frare, C. and Rice, S. A. (2017). Neural signaling metabolites may modulate energy use in hibernation. Neurochem. Res. 42, 141-150. https://doi.org/10.1007/s11064-016-2109-4Google ScholarFeketa, V. V., Nikolaev, Y. A., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2020). CNGA3 acts as a cold sensor in hypothalamic neurons. Elife 9, e55370. https://doi.org/10.7554/eLife.55370.sa2Google ScholarFeng, N. Y., Junkins, M. S., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2019). Osmolyte depletion and thirst suppression allow hibernators to survive for months without water. Curr. Biol. 29, 3053-3058.e3. https://doi.org/10.1016/j.cub.2019.07.038Google ScholarFlorant, G. L., Fenn, A. M., Healy, J. E., Wilkerson, G. K. and Handa, R. J. (2010). To eat or not to eat: the effect of AICAR on food intake regulation in yellow-bellied marmots (Marmota flaviventris). J. Exp. Biol. 213, 2031-2037. https://doi.org/10.1242/jeb.039131Google ScholarFrare, C. and Drew, K. L. (2021). Seasonal changes in adenosine kinase in tanycytes of the Arctic ground squirrel (Urocitellus parryii). J. Chem. Neuroanat. 113, 101920. https://doi.org/10.1016/j.jchemneu.2021.101920Google ScholarFrare, C., Williams, C. T. and Drew, K. L. (2021). Thermoregulation in hibernating mammals: The role of the “thyroid hormones system”. Mol. Cell. Endocrinol. 519, 111054. https://doi.org/10.1016/j.mce.2020.111054Google ScholarGalic, S., Loh, K., Murray-Segal, L., Steinberg, G. R., Andrews, Z. B. and Kemp, B. E. (2018). AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. Elife 7, e32656. https://doi.org/10.7554/eLife.32656.030Google ScholarHamilton, J. D. and Pfeiffer, E. W. (1977). Effects of cold exposure and dehydration on renal function in black-tailed prairie dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 42, 295-299. https://doi.org/10.1152/jappl.1977.42.2.295Google ScholarHealy, J. E., Bateman, J. L., Ostrom, C. E. and Florant, G. L. (2011). Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm. Behav. 59, 512-519. https://doi.org/10.1016/j.yhbeh.2011.01.013Google ScholarHealy, J. E., Ostrom, C. E., Wilkerson, G. K. and Florant, G. L. (2010). Plasma ghrelin concentrations change with physiological state in a sciurid hibernator (Spermophilus lateralis). Gen. Comp. Endocrinol. 166, 372-378. https://doi.org/10.1016/j.ygcen.2009.12.006Google ScholarHengen, K. B., Behan, M., Carey, H. V., Jones, M. V. and Johnson, S. M. (2009). Hibernation induces pentobarbital insensitivity in medulla but not cortex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1028-R1036. https://doi.org/10.1152/ajpregu.00239.2009Google ScholarHengen, K. B., Gomez, T. M., Stang, K. M., Johnson, S. M. and Behan, M. (2011). Changes in ventral respiratory column GABAaR epsilon- and delta-subunits during hibernation mediate resistance to depression by EtOH and pentobarbital. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R272-R283. https://doi.org/10.1152/ajpregu.00607.2010Google ScholarHerwig, A., Wilson, D., Logie, T. J., Boelen, A., Morgan, P. J., Mercer, J. G. and Barrett, P. (2009). Photoperiod and acute energy deficits interact on components of the thyroid hormone system in hypothalamic tanycytes of the Siberian hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1307-R1315. https://doi.org/10.1152/ajpregu.90755.2008Google ScholarHorwitz, B. A., Chau, S. M., Hamilton, J. S., Song, C., Gorgone, J., Saenz, M., Horowitz, J. M. and Chen, C.-Y. (2013). Temporal relationships of blood pressure, heart rate, baroreflex function, and body temperature change over a hibernation bout in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R759-R768. https://doi.org/10.1152/ajpregu.00450.2012Google ScholarHrvatin, S., Sun, S., Wilcox, O. F., Yao, H., Lavin-Peter, A. J., Cicconet, M., Assad, E. G., Palmer, M. E., Aronson, S., Banks, A. S. et al. (2020). Neurons that regulate mouse torpor. Nature 583, 115-121.Google ScholarHuo, L., Gao, Y., Zhang, D., Wang, S., Han, Y., Men, H., Yang, Z., Qin, X., Wang, R., Kong, D. et al. (2021). Piezo2 channel in nodose ganglia neurons is essential in controlling hypertension in a pathway regulated directly by Nedd4-2. Pharmacol. Res. 164, 105391. https://doi.org/10.1016/j.phrs.2020.105391Google ScholarJani, A., Orlicky, D. J., Karimpour-Fard, A., Epperson, L. E., Russell, R. L., Hunter, L. E. and Martin, S. L. (2012). Kidney proteome changes provide evidence for a dynamic metabolism and regional redistribution of plasma proteins during torpor-arousal cycles of hibernation. Physiol. Genomics 44, 717-727. https://doi.org/10.1152/physiolgenomics.00010.2012Google ScholarJinka, T. R., Toien, O. and Drew, K. L. (2011). Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J. Neurosci. 31, 10752-10758. https://doi.org/10.1523/JNEUROSCI.1240-11.2011Google ScholarJinka, T. R., Rasley, B. T. and Drew, K. L. (2012). Inhibition of NMDA-type glutamate receptors induces arousal from torpor in hibernating arctic ground squirrels (Urocitellus parryii). J. Neurochem. 122, 934-940. https://doi.org/10.1111/j.1471-4159.2012.07832.xGoogle ScholarKastner, P. R., Zatzman, M. L., South, F. E. and Johnson, J. A. (1978). Renin-angiotensin-aldosterone system of the hibernating marmot. Am. J. Physiol. 234, R178-R182. https://doi.org/10.1152/ajpregu.1978.234.5.R178Google ScholarKim, A. B. and Milsom, W. K. (2019). pH regulation in hibernation: Implications for ventilatory and metabolic control. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 237, 110536. https://doi.org/10.1016/j.cbpa.2019.110536Google ScholarKohno, D., Sone, H., Minokoshi, Y. and Yada, T. (2008). Ghrelin raises [Ca2+]I via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 366, 388-392. https://doi.org/10.1016/j.bbrc.2007.11.166Google ScholarKramarova, L. I., Kolaeva, S. H., Yukhananov, R. and Rozhanets, V. V. (1983). Content of DSIP, enkephalins and ACTH in some tissues of active and hibernating ground squirrels (Citellus suslicus). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 74, 31-33. https://doi.org/10.1016/0742-8413(83)90143-3Google ScholarKromer, W. (1980). Naltrexone influence on hibernation. Experientia 36, 581-582. https://doi.org/10.1007/BF01965814Google ScholarLage, R., Vázquez, M. J., Varela, L., Saha, A. K., Vidal-Puig, A., Nogueiras, R., Diéguez, C. and López, M. (2010). Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 24, 2670-2679. https://doi.org/10.1096/fj.09-150672Google ScholarLanglet, F., Levin, B. E., Luquet, S., Mazzone, M., Messina, A., Dunn-Meynell, A. A., Balland, E., Lacombe, A., Mazur, D., Carmeliet, P. et al. (2013). Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 17, 607-617. https://doi.org/10.1016/j.cmet.2013.03.004Google ScholarLaursen, W. J., Mastrotto, M., Pesta, D., Funk, O. H., Goodman, J. B., Merriman, D. K., Ingolia, N., Shulman, G. I., Bagriantsev, S. N. and Gracheva, E. O. (2015). Neuronal UCP1 expression suggests a mechanism for local thermogenesis during hibernation. Proc. Natl. Acad. Sci. USA 112, 1607-1612. https://doi.org/10.1073/pnas.1421419112Google ScholarLewis, J. E. and Ebling, F. J. (2017). Tanycytes as regulators of seasonal cycles in neuroendocrine function. Front. Neurol. 8, 79. https://doi.org/10.3389/fneur.2017.00079Google ScholarLyman, C. P. and O'Brien, R. C. (1961). Circulatory changes in the 13-lined ground squirrel during the hibernating cycle. Tech. Rep. Arct. Aeromed. Lab. US 60, 1-18. https://doi.org/10.21236/AD0261757Google ScholarLyman, C. P. and O'Brien, R. C. (1963). Autonomic control of circulation during the hibernating cycle in ground squirrels. J. Physiol. 168, 477-499. https://doi.org/10.1113/jphysiol.1963.sp007204Google ScholarMann, F. C. (1916). The ductless glands and hibernation. Am. J. Physiol. 41, 173-188. https://doi.org/10.1152/ajplegacy.1916.41.2.173Google ScholarMargules, D. L., Goldman, B. and Finck, A. (1979). Hibernation: an opioid-dependent state? Brain Res. Bull. 4, 721-724. https://doi.org/10.1016/0361-9230(79)90003-0Google ScholarMatos-Cruz, V., Schneider, E. R., Mastrotto, M., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2017). Molecular prerequisites for diminished cold sensitivity in ground squirrels and hamsters. Cell Rep 21, 3329-3337. https://doi.org/10.1016/j.celrep.2017.11.083Google ScholarMcArthur, M. D. and Milsom, W. K. (1991). Changes in ventilation and respiratory sensitivity associated with hibernation in columbian (Spermophilus columbianus) and golden-mantled (Spermophilus lateralis) ground squirrels. Physiol. Zool. 64, 940-959. https://doi.org/10.1086/physzool.64.4.30157950Google ScholarMettler, F. A., Mettler, C. C. and Culler, E. (1935). Effects of total removal of the cerebral cortex. Arch. Neurol. Psychiatry 34, 1238-1249. https://doi.org/10.1001/archneurpsyc.1935.02250240107008Google ScholarMilsom, W. K., Harris, M. B. and Reid, S. G. (1997). Do descending influences alternate to produce episodic breathing? Respir. Physiol. 110, 307-317. https://doi.org/10.1016/S0034-5687(97)00096-0Google ScholarMilsom, W. K., Zimmer, M. B. and Harris, M. B. (1999). Regulation of cardiac rhythm in hibernating mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 124, 383-391. https://doi.org/10.1016/S1095-6433(99)00130-0Google ScholarMilsom, W. K., Zimmer, M. B. and Harris, M. B. (2001). Vagal control of cardiorespiratory function in hibernation. Exp. Physiol. 86, 791-796. https://doi.org/10.1111/j.1469-445X.2001.tb00046.xGoogle ScholarMin, S., Chang, R. B., Prescott, S. L., Beeler, B., Joshi, N. R., Strochlic, D. E. and Liberles, S. D. (2019). Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep 29, 2192-2201.e3. https://doi.org/10.1016/j.celrep.2019.10.040Google ScholarMohr, S. M., Bagriantsev, S. N. and Gracheva, E. O. (2020). Cellular, molecular, and physiological adaptations to hibernation: the solution to environmental challenges. Annu. Rev. Cell Dev. Biol. 36, 315-338. https://doi.org/10.1146/annurev-cellbio-012820-095945Google ScholarMorrison, S. F. and Nakamura, K. (2011). Central neural pathways for thermoregulation. Front. Biosci. 16, 74-104. https://doi.org/10.2741/3677Google ScholarMulawa, E. A., Kirkwood, J. S., Wolfe, L. M., Wojda, S. J., Prenni, J. E., Florant, G. L. and Donahue, S. W. (2018). Seasonal changes in endocannabinoid concentrations between active and hibernating marmots (Marmota flaviventris). J. Biol. Rhythms 33, 388-401. https://doi.org/10.1177/0748730418777660Google ScholarNilaweera, K., Herwig, A., Bolborea, M., Campbell, G., Mayer, C. D., Morgan, P. J., Ebling, F. J. and Barrett, P. (2011). Photoperiodic regulation of glycogen metabolism, glycolysis, and glutamine synthesis in tanycytes of the Siberian hamster suggests novel roles of tanycytes in hypothalamic function. Glia 59, 1695-1705. https://doi.org/10.1002/glia.21216Google ScholarPassmore, J. C., Pfeiffer, E. W. and Templeton, J. R. (1975). Urea excretion in the hibernating Columbian ground squirrel (Spermophilus columbianus). J. Exp. Zool. 192, 83-86. https://doi.org/10.1002/jez.1401920110Google ScholarPicardo, M. A., Merel, J., Katlowitz, K. A., Vallentin, D., Okobi, D. E., Benezra, S. E., Clary, R. C., Pnevmatikakis, E. A., Paninski, L. and Long, M. A. (2016). Population-level representation of a temporal sequence underlying song production in the Zebra finch. Neuron 90, 866-876. https://doi.org/10.1016/j.neuron.2016.03.038Google ScholarProvince, H. S., Xiao, C., Mogul, A. S., Sahoo, A., Jacobson, K. A., Piñol, R. A., Gavrilova, O. and Reitman, M. L. (2020). Activation of neuronal adenosine A1 receptors causes hypothermia through central and peripheral mechanisms. PLoS ONE 15, e0243986. https://doi.org/10.1371/journal.pone.0243986Google ScholarRasmussen, A. T. (1916). Theories of hibernation. Am. Nat. 50, 609-625. https://doi.org/10.1086/279571Google ScholarSallmen, T., Beckman, A. L., Stanton, T. L., Eriksson, K. S., Tarhanen, J., Tuomisto, L. and Panula, P. (1999). Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J. Neurosci. 19, 1824-1835. https://doi.org/10.1523/JNEUROSCI.19-05-01824.1999Google ScholarSaper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. and Scammell, T. E. (2010). Sleep state switching. Neuron 68, 1023-1042. https://doi.org/10.1016/j.neuron.2010.11.032Google ScholarSatinoff, E. (1964). Behavioral thermoregulation in response to local cooling of the rat brain. Am. J. Physiol. 206, 1389-1394. https://doi.org/10.1152/ajplegacy.1964.206.6.1389Google ScholarSchwartz, C., Hampton, M. and Andrews, M. T. (2013). Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS ONE 8, e58427. https://doi.org/10.1371/journal.pone.0058427Google ScholarSchwartz, C., Hampton, M. and Andrews, M. T. (2015). Hypothalamic gene expression underlying pre-hibernation satiety. Genes Brain Behav. 14, 310-318. https://doi.org/10.1111/gbb.12199Google ScholarScribner, J. L., Vance, E. A., Protter, D. S. W., Sheeran, W. M., Saslow, E., Cameron, R. T., Klein, E. M., Jimenez, J. C., Kheirbek, M. A. and Donaldson, Z. R. (2020). A neuronal signature for monogamous reunion. Proc. Natl. Acad. Sci. USA 117, 11076-11084. https://doi.org/10.1073/pnas.1917287117Google ScholarSekizawa, S., Bechtold, A. G., Tham, R. C. and Bonham, A. C. (2009). A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J. Neurosci. 29, 11807-11816. https://doi.org/10.1523/JNEUROSCI.2617-09.2009Google ScholarSekizawa, S., Horwitz, B. A., Horowitz, J. M. and Chen, C.-Y. (2013). Protection of signal processing at low temperature in baroreceptive neurons in the nucleus tractus solitarius of Syrian hamsters, a hibernating species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R1153-R1162. https://doi.org/10.1152/ajpregu.00165.2013Google ScholarSilvani, A., Cerri, M., Zoccoli, G. and Swoap, S. J. (2018). Is adenosine action common ground for NREM sleep, torpor, and other hypometabolic states? Physiology 33, 182-196. https://doi.org/10.1152/physiol.00007.2018Google ScholarSingh Alvarado, J., Goffinet, J., Michael, V., Liberti, W., III, Hatfield, J., Gardner, T., Pearson, J. and Mooney, R. (2021). Neural dynamics underlying birdsong practice and performance. Nature 599, 635-639. https://doi.org/10.1038/s41586-021-04004-1Google ScholarStrumwasser, F. (1958). Regulatory mechanisms, brain activity and behavior during deep hibernation in the squirrel, Citellus beecheyi. Am. J. Physiol. 196, 23-30. https://doi.org/10.1152/ajplegacy.1958.196.1.23Google ScholarTakahashi, T. M., Sunagawa, G. A., Soya, S., Abe, M., Sakurai, K., Ishikawa, K., Yanagisawa, M., Hama, H., Hasegawa, E., Miyawaki, A., et al. (2020). A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583, 109-114.Google ScholarTamura, Y., Shintani, M., Nakamura, A., Monden, M. and Shiomi, H. (2005). Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 1045, 88-96. https://doi.org/10.1016/j.brainres.2005.03.029Google ScholarTan, C. L. and Knight, Z. A. (2018). Regulation of body temperature by the nervous system. Neuron 98, 31-48. https://doi.org/10.1016/j.neuron.2018.02.022Google ScholarTorke, K. G. and Twente, J. W. (1977). Behavior of Spermophilus lateralis between periods of hibernation. J. Mammal. 58, 385-390. https://doi.org/10.2307/1379337Google ScholarTupone, D., Madden, C. J. and Morrison, S. F. (2013). Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J. Neurosci. 33, 14512-14525. https://doi.org/10.1523/JNEUROSCI.1980-13.2013Google ScholarTupone, D., Cano, G. and Morrison, S. F. (2017). Thermoregulatory inversion: a novel thermoregulatory paradigm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R779-R786. https://doi.org/10.1152/ajpregu.00022.2017Google ScholarWalker, J. M., Glotzbach, S. F., Berger, R. J. and Heller, H. C. (1977). Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am. J. Physiol. 233, R213-R221. https://doi.org/10.1152/ajpregu.1977.233.5.R213Google ScholarYackle, K., Schwarz, L. A., Kam, K., Sorokin, J. M., Huguenard, J. R., Feldman, J. L., Luo, L. and Krasnow, M. A. (2017). Breathing control center neurons that promote arousal in mice. Science 355, 1411-1415. https://doi.org/10.1126/science.aai7984Google ScholarZatzman, M. L. and South, F. E. (1981). Circannual renal function and plasma electrolytes of the marmot. Am. J. Physiol. 241, R87-R91. https://doi.org/10.1152/ajpregu.1981.241.1.R87Google ScholarZeng, W.-Z., Marshall, K. L., Min, S., Daou, I., Chapleau, M. W., Abboud, F. M., Liberles, S. D. and Patapoutian, A. (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362, 464-467. https://doi.org/10.1126/science.aau6324Google ScholarZimmerman, C. A., Lin, Y.-C., Leib, D. E., Guo, L., Huey, E. L., Daly, G. E., Chen, Y. and Knight, Z. A. (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680-684. https://doi.org/10.1038/nature18950Google ScholarZimmerman, C. A., Leib, D. E. and Knight, Z. A. (2017). Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18, 459-469. https://doi.org/10.1038/nrn.2017.71Google ScholarZimny, M. L., Franco, E. E., St Onge, M. and Pearson, J. (1984). Ultrastructure of juxtaglomerular cells correlated with biochemical parameters in a hibernator. Comp. Biochem. Physiol. A Comp. Physiol. 78, 229-235. https://doi.org/10.1016/0300-9629(84)90137-3
Thermoregulatory responses to cold. Hibernators express cold-insensitive orthologs of TRPM8 in dorsal root ganglion (DRG) neurons and CNGA3 in preoptic area (POA) hypothalamic neurons. Additionally, there are fewer cold-sensitive neurons in the POA of hibernators versus non-hibernators. Thus, DRG and POA neurons from hibernators cannot respond as strongly to cold exposure. Neuronal response to cold is represented in light blue; insensitivity to cold in black. In non-hibernators, hypothalamic cooling is sufficient to trigger a thermoregulatory response (Satinoff, 1964). By contrast, hibernators exhibit extremely low brain and body temperatures in torpor, suggesting that thermoregulatory suppression is supported by the CNS in addition to the PNS mechanism described above. Research in thirteen-lined ground squirrels has provided some insight into the role of the CNS in this process. Compared with mice, squirrels have fewer intrinsically cold-sensitive POA neurons (Feketa et al., 2020). Although the molecular basis of cold sensitivity in POA neurons remains unclear, it was suggested that it is partially mediated by CNGA3, a non-selective excitatory cyclic nucleotide gated ion channel present in POA neurons of mice and squirrels. The activity of mouse CNGA3 is robustly potentiated by cooling; however, no such potentiation is observed in the squirrel ortholog (Feketa et al., 2020). Thus, CNS cold sensors permit thermoregulatory suppression during torpor in a manner analogous to the PNS mechanism (Fig. 3). It is worth noting that in rats, which do not hibernate, silencing output from POA neurons can trigger a unique state known as thermoregulatory inversion, in which cold exposure facilitates a decrease in body temperature instead of an increase (Tupone et al., 2017). As such, thermoregulatory inversion appears to mimic some features of hibernation; however, the extent to which these phenomena overlap remains to be determined. In summary, it is likely that hibernators possess an impaired ability to sense the decreases in brain and body temperature that would normally trigger a thermogenic response, thereby enabling heterothermy. Brainstem control of homeostasis Brainstem nuclei promote other forms of homeostasis by coordinating autonomic responses. These include robust cardiovascular and pulmonary reflexes, which must be quelled during the hibernation season to prevent premature emergence. During entry into torpor, for example, blood pressure and heart rate drop concomitantly (Chatfield and Lyman, 1950; Lyman and O'Brien, 1961; Horwitz et al., 2013). This phenomenon is indicative of dramatic changes to autonomic processes designed to compensate for blood pressure fluctuations with adjustments to heart rate (Zeng et al., 2018). Significant insights have been made into the molecular mechanisms protecting hibernators from ischemia, reperfusion and hypoxia (Dave et al., 2012), but considerably less is known about how the nervous system permits these conditions in the first place. Cardiovascular function The autonomic nervous system (ANS) regulates blood pressure and heart rate to maintain precise control over cardiovascular function (Boron and Boulpaep, 2016). In the case of a drop in blood pressure, mechanosensitive baroreceptor neurons sense a decrease in arterial stretch, resulting in a lower firing frequency. These neurons project via the glossopharyngeal nerve to the brainstem. A reduction in firing rate indirectly engages an increase in sympathetic tone. Sympathetic efferents deliver norepinephrine to the cardiovascular system, promoting vasoconstriction, tachycardia and increased contractility of cardiac muscle, and resulting in a near-instantaneous elevation in blood pressure. Parasympathetic efferents, which rely on acetylcholine to slow cardiovascular function, are concurrently inhibited (Boron and Boulpaep, 2016). In stark contrast to non-hibernators, animals entering torpor exhibit a simultaneous, precipitous drop in heart rate and blood pressure that precedes the decrease in body temperature (Chatfield and Lyman, 1950; Horwitz et al., 2013; Lyman and O'Brien, 1961). In Gould's long-eared bats, heart rate and metabolic rate are positively correlated in daily torpor, but in a manner that is distinct from their euthermic conspecifics (Currie et al., 2014). This finding supports the notion that the cardiovascular system behaves differently during periods of heterothermy. Torpor continues for days or weeks with remarkably subdued cardiac function, indicating that the ANS is permissive of this state. The baroreceptor reflex is almost completely suppressed in torpid Syrian hamsters and remains so until late into the ensuing IBA (Horwitz et al., 2013). However, during entry into torpor, the sensitivity of the baroreceptor reflex remains high, suggesting that it plays an active role in the initiation process, perhaps by adjusting the set point for BP (Horwitz et al., 2013). The mechanisms underlying changes to the baroreceptor reflex in hibernation are not known. One possibility is the alteration of baroreceptor sensitivity, e.g. via changes in the function of Piezo1 and Piezo2, mechanically gated ion channels that can detect arterial stretch (Huo et al., 2021; Min et al., 2019; Zeng et al., 2018). Another is the alteration in baroreceptor signaling. Baroreceptor neurons in the carotid artery synapse onto ‘second-order’ neurons residing in the nucleus of the solitary tract (NTS) (Sekizawa et al., 2009) (Fig. 2). One group reported that NTS neurons exhibited different electrophysiological properties in euthermic versus torpid Syrian hamsters (Sekizawa et al., 2013). Although neurons from both states had similar resting membrane potentials, NMDA-induced currents were smaller in torpid neurons at membrane voltages lower than −30 mV. Furthermore, NMDA receptor-independent inputs from first-order baroreceptor neurons from hibernating hamsters resulted in excitatory postsynaptic currents (EPSCs) that decayed more quickly when compared with EPSCs in neurons from euthermic hamsters. These changes suggest a difference in signaling between baroreceptors and second-order neurons in NTS across the hibernation cycle. The baroreceptor reflex engages the parasympathetic (PSNS) and sympathetic nervous (SNS) systems to maintain bidirectional control over heart rate, and there is pharmacological evidence that both systems may be greatly altered during hibernation. PSNS blockade with veratramine, atropine or via vagotomy does not trigger the expected increase in heart rate in torpid thirteen-lined tree squirrels, indicating that the PSNS is almost completely suppressed (Lyman and O'Brien, 1963; Milsom et al., 1999). Supporting this, the SNS agonist norepinephrine elicits hypertension and compensatory bradycardia in active ground squirrels, whereas the same treatment during torpor paradoxically triggers tachycardia (Lyman and O'Brien, 1961). Since the PSNS is responsible for slowing the heart rate, this result serves as indirect evidence of its suppression while the SNS remains partly intact. However, although torpid ground squirrels are still responsive to SNS antagonists in a β-adrenergic receptor-dependent manner, SNS activity is likely to be greatly suppressed compared with levels in an active animal because heart rate and blood pressure are so low (Milsom et al., 2001). Pulmonary function The respiratory system serves to maintain the appropriate levels of blood oxygenation and blood acidity. Deviations from these set points are sensed by peripheral chemoreceptors, which fire action potentials in response to hypoxia, hypercarbia and low pH (Boron and Boulpaep, 2016). This information is conveyed by several cranial nerves to respiratory neurons in the medulla oblongata, including neurons of the Bötzinger and pre-Bötzinger complexes, and rostral division of the ventral respiratory group (Fig. 2). There, a sensory subpopulation receives input from the peripheral chemoreceptors and projects to premotor and motor populations, which coordinate an appropriate breathing response (Boron and Boulpaep, 2016). Of note, a specialized set of premotor neurons is also involved in the modulation of arousal states in mice (Yackle et al., 2017). Within torpor, hibernators may breathe as infrequently as once per minute, suggesting that the respiratory system is radically altered (McArthur and Milsom, 1991). In golden-mantled ground squirrels, this is in part a passive consequence accompanying the reduction in respiratory drive, since metabolic rate is suppressed and blood pH becomes slightly elevated in several species (Kim and Milsom, 2019), but evidence suggests that the neural control of respiratory rhythm is also actively modulated. Many hibernators exhibit a pattern of episodic breathing characterized by clusters of fast, evenly spaced breaths interspersed with prolonged apneas (McArthur and Milsom, 1991; Milsom et al., 2001). This is in stark contrast to euthermic mammals, in which respiratory rhythm remains constant. Whereas euthermic mammals usually alter breath volume and duration to adjust blood oxygenation and pH, torpid golden-mantled ground squirrels instead adjust breath frequency and apnea duration (Milsom et al., 2001). Neither the physiological significance nor the neural mechanisms underlying the different breathing patterns are known, but there are some clues. One study revealed that cooling below 4°C restored evenly spaced breathing in torpid golden-mantled ground squirrels (Milsom et al., 1997). The authors posit that the disappearance of apneas and clustered high-frequency breaths is due to the removal of inhibitory and excitatory inputs, respectively, leaving only the basic pattern generated by respiratory neurons. Independently, neither pharmacological blockade of NMDA receptors nor vagotomy eliminated episodic breathing. However, combining both approaches led to evenly spaced breaths and rapid emergence from torpor (Milsom et al., 1997). Thus, it is possible that some combination of sensory input from the vagus nerve and glutamatergic input to respiratory neurons is required for the unusual rhythm observed in torpor. Recent work has revealed that changes in the expression of GABA¬A receptor subunits across the hibernation cycle produce functional differences in respiratory neurons, hinting at the presence of other molecular mechanisms (Hengen et al., 2009, 2011). Neuromodulation in hibernation We have discussed how hibernation is facilitated and reinforced by significant alterations to brainstem and hypothalamus function. The process by which these changes come about is not known, but evidence supports the involvement of several neuromodulators, including adenosine, endorphins, histamine, thyroid hormone, endocannabinoids and glutamate (Beckman et al., 1981; Frare et al., 2021; Jinka et al., 2012, 2011; Mulawa et al., 2018; Sallmen et al., 1999). By exploring the complex interactions between neuromodulatory systems in hibernation, the field of hibernation research may approach its major goal of determining whether it is possible to induce synthetic torpor in non-hibernators. Adenosine Adenosine is known to be important for the neural control of sleep, and recent work suggests that it may have similar significance for the induction and maintenance of hibernation (Silvani et al., 2018). As an essential component of the ATP pathway, this molecule may serve as a readout of metabolic state (Tupone et al., 2013). In the context of sleep, adenosine accumulates in the basal forebrain throughout the day, activating neurons in the ventrolateral preoptic area of the hypothalamus (VLPO), which in turn inhibit subcortical neurotransmission associated with waking behavior (Drew et al., 2017; Saper et al., 2010). Although it is not known whether a similar accumulation of adenosine occurs circannually in obligate hibernators, there is evidence that this neuromodulator is involved in torpor entry and maintenance. Intracerebroventricular administration of an adenosine A1 receptor (A1AR) antagonist prevents torpor entry in AGS and induces emergence from hibernation in Syrian hamsters (Jinka et al., 2011; Tamura et al., 2005). Furthermore, in AGS, the A1AR agonist N6-cyclohexyladenosine (CHA) promotes torpor entry (Jinka et al., 2011). The effect is only apparent in the winter, suggesting that other seasonal changes are required to sensitize the brain to adenosine. In the rat, which does not hibernate, intracerebroventricular CHA delivery in combination with cooling of environmental temperature significantly lowers body temperature, heart rate and electroencephalogram amplitude, inhibits thermogenesis and can also induce a state of thermoregulatory inversion mentioned above (see ‘Thermogenesis’ section; Tupone et al., 2013, 2017). The activation of A1AR+ neurons in the brainstem nucleus of the solitary tract (NTS) is sufficient to induce this torpor-like state. This treatment increases blood pressure and parasympathetic tone (Tupone et al., 2013), suggesting that it does not recapitulate every feature of hibernation (see ‘Cardiovascular function’ section). Nonetheless, this work highlights A1AR+ NTS neurons as intriguing candidates to study in hibernators. It is also important to note that other studies in non-hibernators have found that CHA-induced hypothermia may occur independently of central A1AR activation (Province et al., 2020). This discrepancy underscores the need for further investigation of adenosinergic signaling in hypothermic states, including hibernation. Endorphins Evidence suggests that hibernation is promoted by the action of endogenous opioids, known as endorphins. These neuropeptides are perhaps most famous for their role in masking pain following strenuous exercise, but they have been implicated in a wide array of physiological processes. Their neuromodulatory effect is enacted through opioid receptors distributed widely throughout the brain (Bourhim et al., 1997). The activation of mu, kappa and delta opioid receptors have been associated with changes in appetite, thirst, body temperature and physical activity similar to those seen across the hibernation cycle (Bodnar, 2020). High concentrations of endorphins have been detected in the brains of hibernators during the winter (Cui et al., 1996; Kramarova et al., 1983). Additionally, the administration of opioid receptor antagonists, including naloxone, is known to interfere with hibernation maintenance in several species (Beckman and Llados-Eckman, 1985; Kromer, 1980; Margules et al., 1979; Tamura et al., 2005). The activation of opioid receptors is known to elicit effects often associated with hibernation, such as analgesia, hypothermia, bradycardia and slow breathing rate (Ban et al., 2020; Bodnar, 2020; Cintron-Colon et al., 2019). It is thus tempting to speculate that endorphinergic signaling is required for hibernation in several species. However, the field has yet to establish a connection between the molecular actions of endorphins with the neurophysiological changes that occur in hibernation. Conclusion In the past two centuries, researchers have made great progress towards understanding the phenomenon of hibernation. Accumulated evidence strongly suggests that the hypothalamus and brainstem play an essential role in the process by adjusting homeostatic set points, and this knowledge highlights several exciting research directions. Modern neuroscience tools will allow us to clearly define the neural underpinnings of the hibernation phenotype, drawing us nearer to unlocking these mechanisms in non-hibernators. Opto- and chemogenetic manipulations, as well as in vivo calcium imaging methods, have been successfully applied in non-standard animal models such as the prairie vole (Amadei et al., 2017; Scribner et al., 2020) and the zebra finch (Picardo et al., 2016; Singh Alvarado et al., 2021). Contemporaneous studies have identified discrete hypothalamic neuronal populations that regulate fasting-induced torpor in mice (Hrvatin et al., 2020) and a hibernation-like state in rats (Takahashi et al., 2020). Going forward, it will be essential to manipulate these discrete neuronal populations, and the other neural circuits mentioned in this Review, using opto- or chemogenetics in true hibernators to determine their necessity and sufficiency in this complex physiological phenomenon. The stage is set for a new era of research into the neurobiology of hibernation. ReferencesAlhadeff, A. L., Su, Z., Hernandez, E., Klima, M. L., Phillips, S. Z., Holland, R. A., Guo, C., Hantman, A. W., De Jonghe, B. C. and Betley, J. N. (2018). A neural circuit for the suppression of pain by a competing need state. Cell 173, 140-152.e15. https://doi.org/10.1016/j.cell.2018.02.057Google ScholarAmadei, E. A., Johnson, Z. V., Jun Kwon, Y., Shpiner, A. C., Saravanan, V., Mays, W. D., Ryan, S. J., Walum, H., Rainnie, D. G., Young, L. J. et al. (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297-301. https://doi.org/10.1038/nature22381Google ScholarAndersen, P., Johansen, K., Krog, J. (1960). Electroencephalogram during arousal from hibernation in the birchmouse. Am. J. Physiol. 199, 535-538. https://doi.org/10.1152/ajplegacy.1960.199.3.535Google ScholarAndrews, M. T. (2019). Molecular interactions underpinning the phenotype of hibernation in mammals. J. Exp. Biol. 222, jeb160606. https://doi.org/10.1242/jeb.160606Google ScholarAngilletta, M. J., Jr, Youngblood, J. P., Neel, L. K. and VandenBrooks, J. M. (2019). The neuroscience of adaptive thermoregulation. Neurosci. Lett. 692, 127-136. https://doi.org/10.1016/j.neulet.2018.10.046Google ScholarAugustine, V., Gokce, S. K., Lee, S., Wang, B., Davidson, T. J., Reimann, F., Gribble, F., Deisseroth, K., Lois, C. and Oka, Y. (2018). Hierarchical neural architecture underlying thirst regulation. Nature 555, 204-209. https://doi.org/10.1038/nature25488Google ScholarAugustine, V., Lee, S. and Oka, Y. (2020). Neural control and modulation of thirst, sodium appetite, and hunger. Cell 180, 25-32. https://doi.org/10.1016/j.cell.2019.11.040Google ScholarBan, E. G., Brassai, A. and Vizi, E. S. (2020). The role of the endogenous neurotransmitters associated with neuropathic pain and in the opioid crisis: the innate pain-relieving system. Brain Res. Bull. 155, 129-136. https://doi.org/10.1016/j.brainresbull.2019.12.001Google ScholarBarrett, P., Ivanova, E., Graham, E. S., Ross, A. W., Wilson, D., Plé, H., Mercer, J. G., Ebling, F. J., Schuhler, S., Dupré, S. M. et al. (2006). Photoperiodic regulation of cellular retinol binding protein, CRBP1 [corrected] and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J. Endocrinol. 191, 687-698. https://doi.org/10.1677/joe.1.06929Google ScholarBautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I., Stucky, C. L., Jordt, S.-E. and Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204-208. https://doi.org/10.1038/nature05910Google ScholarBeckman, A. L. and Llados-Eckman, C. (1985). Antagonism of brain opioid peptide action reduces hibernation bout duration. Brain Res. 328, 201-205. https://doi.org/10.1016/0006-8993(85)91030-3Google ScholarBeckman, A. L., Llados-Eckman, C., Stanton, T. L. and Adler, M. W. (1981). Physical dependence on morphine fails to develop during the hibernating state. Science 212, 1527-1529. https://doi.org/10.1126/science.7233241Google ScholarBjursten, L. M., Norrsell, K. and Norrsell, U. (1976). Behavioural repertory of cats without cerebral cortex from infancy. Exp. Brain Res. 25, 115-130. https://doi.org/10.1007/BF00234897Google ScholarBlouet, C. and Schwartz, G. J. (2012). Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579-587. https://doi.org/10.1016/j.cmet.2012.10.003Google ScholarBodnar, R. J. (2020). Endogenous opiates and behavior: 2017. Peptides 124, 170223. https://doi.org/10.1016/j.peptides.2019.170223Google ScholarBolborea, M., Pollatzek, E., Benford, H., Sotelo-Hitschfeld, T. and Dale, N. (2020). Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc. Natl. Acad. Sci. USA 117, 14473-14481. https://doi.org/10.1073/pnas.1919887117Google ScholarBoron, W. F. and Boulpaep, E. L. (2016). Boron & Boulpaep Concise Medical Physiology. Elsevier Health Sciences.Google ScholarBourhim, N., Kabine, M. and Elkebbaj, M. S. (1997). Characterization of opioid peptides and opioid receptors in the brain of jerboa (Jaculus orientalis), a hibernating rodent. Brain Res. Bull. 44, 615-620. https://doi.org/10.1016/S0361-9230(97)00282-7Google ScholarCaterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816-824. https://doi.org/10.1038/39807Google ScholarChatfield, P. O. and Lyman, C. P. (1950). Circulatory changes during process of arousal in the hibernating hamster. Am. J. Physiol. 163, 566-574. https://doi.org/10.1152/ajplegacy.1950.163.3.566Google ScholarCintron-Colon, R., Johnson, C. W., Montenegro-Burke, J. R., Guijas, C., Faulhaber, L., Sanchez-Alavez, M., Aguirre, C. A., Shankar, K., Singh, M., Galmozzi, A. et al. (2019). Activation of kappa opioid receptor regulates the hypothermic response to calorie restriction and limits body weight loss. Curr. Biol. 29, 4291-4299.e4. https://doi.org/10.1016/j.cub.2019.10.027Google ScholarCui, Y., Lee, T. F. and Wang, L. C. (1996). State-dependent changes of brain endogenous opioids in mammalian hibernation. Brain Res. Bull. 40, 129-133. https://doi.org/10.1016/0361-9230(96)00038-XGoogle ScholarCurrie, S. E., Körtner, G. and Geiser, F. (2014). Heart rate as a predictor of metabolic rate in heterothermic bats. J. Exp. Biol. 217, 1519-1524. https://doi.org/10.1242/jeb.098970Google ScholarDave, K. R., Christian, S. L., Perez-Pinzon, M. A. and Drew, K. L. (2012). Neuroprotection: lessons from hibernators. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 162, 1-9. https://doi.org/10.1016/j.cbpb.2012.01.008Google ScholarDavern, P. J. (2014). A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Front. Physiol. 5, 436. https://doi.org/10.3389/fphys.2014.00436Google ScholarD'Alessandro, A., Nemkov, T., Bogren, L. K., Martin, S. L. and Hansen, K. C. (2017). comfortably numb and back: plasma metabolomics reveals biochemical adaptations in the hibernating 13-lined ground squirrel. J. Proteome Res. 16, 958-969. https://doi.org/10.1021/acs.jproteome.6b00884Google ScholarDrew, K. L., Frare, C. and Rice, S. A. (2017). Neural signaling metabolites may modulate energy use in hibernation. Neurochem. Res. 42, 141-150. https://doi.org/10.1007/s11064-016-2109-4Google ScholarFeketa, V. V., Nikolaev, Y. A., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2020). CNGA3 acts as a cold sensor in hypothalamic neurons. Elife 9, e55370. https://doi.org/10.7554/eLife.55370.sa2Google ScholarFeng, N. Y., Junkins, M. S., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2019). Osmolyte depletion and thirst suppression allow hibernators to survive for months without water. Curr. Biol. 29, 3053-3058.e3. https://doi.org/10.1016/j.cub.2019.07.038Google ScholarFlorant, G. L., Fenn, A. M., Healy, J. E., Wilkerson, G. K. and Handa, R. J. (2010). To eat or not to eat: the effect of AICAR on food intake regulation in yellow-bellied marmots (Marmota flaviventris). J. Exp. Biol. 213, 2031-2037. https://doi.org/10.1242/jeb.039131Google ScholarFrare, C. and Drew, K. L. (2021). Seasonal changes in adenosine kinase in tanycytes of the Arctic ground squirrel (Urocitellus parryii). J. Chem. Neuroanat. 113, 101920. https://doi.org/10.1016/j.jchemneu.2021.101920Google ScholarFrare, C., Williams, C. T. and Drew, K. L. (2021). Thermoregulation in hibernating mammals: The role of the “thyroid hormones system”. Mol. Cell. Endocrinol. 519, 111054. https://doi.org/10.1016/j.mce.2020.111054Google ScholarGalic, S., Loh, K., Murray-Segal, L., Steinberg, G. R., Andrews, Z. B. and Kemp, B. E. (2018). AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. Elife 7, e32656. https://doi.org/10.7554/eLife.32656.030Google ScholarHamilton, J. D. and Pfeiffer, E. W. (1977). Effects of cold exposure and dehydration on renal function in black-tailed prairie dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 42, 295-299. https://doi.org/10.1152/jappl.1977.42.2.295Google ScholarHealy, J. E., Bateman, J. L., Ostrom, C. E. and Florant, G. L. (2011). Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm. Behav. 59, 512-519. https://doi.org/10.1016/j.yhbeh.2011.01.013Google ScholarHealy, J. E., Ostrom, C. E., Wilkerson, G. K. and Florant, G. L. (2010). Plasma ghrelin concentrations change with physiological state in a sciurid hibernator (Spermophilus lateralis). Gen. Comp. Endocrinol. 166, 372-378. https://doi.org/10.1016/j.ygcen.2009.12.006Google ScholarHengen, K. B., Behan, M., Carey, H. V., Jones, M. V. and Johnson, S. M. (2009). Hibernation induces pentobarbital insensitivity in medulla but not cortex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1028-R1036. https://doi.org/10.1152/ajpregu.00239.2009Google ScholarHengen, K. B., Gomez, T. M., Stang, K. M., Johnson, S. M. and Behan, M. (2011). Changes in ventral respiratory column GABAaR epsilon- and delta-subunits during hibernation mediate resistance to depression by EtOH and pentobarbital. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R272-R283. https://doi.org/10.1152/ajpregu.00607.2010Google ScholarHerwig, A., Wilson, D., Logie, T. J., Boelen, A., Morgan, P. J., Mercer, J. G. and Barrett, P. (2009). Photoperiod and acute energy deficits interact on components of the thyroid hormone system in hypothalamic tanycytes of the Siberian hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1307-R1315. https://doi.org/10.1152/ajpregu.90755.2008Google ScholarHorwitz, B. A., Chau, S. M., Hamilton, J. S., Song, C., Gorgone, J., Saenz, M., Horowitz, J. M. and Chen, C.-Y. (2013). Temporal relationships of blood pressure, heart rate, baroreflex function, and body temperature change over a hibernation bout in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R759-R768. https://doi.org/10.1152/ajpregu.00450.2012Google ScholarHrvatin, S., Sun, S., Wilcox, O. F., Yao, H., Lavin-Peter, A. J., Cicconet, M., Assad, E. G., Palmer, M. E., Aronson, S., Banks, A. S. et al. (2020). Neurons that regulate mouse torpor. Nature 583, 115-121.Google ScholarHuo, L., Gao, Y., Zhang, D., Wang, S., Han, Y., Men, H., Yang, Z., Qin, X., Wang, R., Kong, D. et al. (2021). Piezo2 channel in nodose ganglia neurons is essential in controlling hypertension in a pathway regulated directly by Nedd4-2. Pharmacol. Res. 164, 105391. https://doi.org/10.1016/j.phrs.2020.105391Google ScholarJani, A., Orlicky, D. J., Karimpour-Fard, A., Epperson, L. E., Russell, R. L., Hunter, L. E. and Martin, S. L. (2012). Kidney proteome changes provide evidence for a dynamic metabolism and regional redistribution of plasma proteins during torpor-arousal cycles of hibernation. Physiol. Genomics 44, 717-727. https://doi.org/10.1152/physiolgenomics.00010.2012Google ScholarJinka, T. R., Toien, O. and Drew, K. L. (2011). Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J. Neurosci. 31, 10752-10758. https://doi.org/10.1523/JNEUROSCI.1240-11.2011Google ScholarJinka, T. R., Rasley, B. T. and Drew, K. L. (2012). Inhibition of NMDA-type glutamate receptors induces arousal from torpor in hibernating arctic ground squirrels (Urocitellus parryii). J. Neurochem. 122, 934-940. https://doi.org/10.1111/j.1471-4159.2012.07832.xGoogle ScholarKastner, P. R., Zatzman, M. L., South, F. E. and Johnson, J. A. (1978). Renin-angiotensin-aldosterone system of the hibernating marmot. Am. J. Physiol. 234, R178-R182. https://doi.org/10.1152/ajpregu.1978.234.5.R178Google ScholarKim, A. B. and Milsom, W. K. (2019). pH regulation in hibernation: Implications for ventilatory and metabolic control. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 237, 110536. https://doi.org/10.1016/j.cbpa.2019.110536Google ScholarKohno, D., Sone, H., Minokoshi, Y. and Yada, T. (2008). Ghrelin raises [Ca2+]I via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 366, 388-392. https://doi.org/10.1016/j.bbrc.2007.11.166Google ScholarKramarova, L. I., Kolaeva, S. H., Yukhananov, R. and Rozhanets, V. V. (1983). Content of DSIP, enkephalins and ACTH in some tissues of active and hibernating ground squirrels (Citellus suslicus). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 74, 31-33. https://doi.org/10.1016/0742-8413(83)90143-3Google ScholarKromer, W. (1980). Naltrexone influence on hibernation. Experientia 36, 581-582. https://doi.org/10.1007/BF01965814Google ScholarLage, R., Vázquez, M. J., Varela, L., Saha, A. K., Vidal-Puig, A., Nogueiras, R., Diéguez, C. and López, M. (2010). Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 24, 2670-2679. https://doi.org/10.1096/fj.09-150672Google ScholarLanglet, F., Levin, B. E., Luquet, S., Mazzone, M., Messina, A., Dunn-Meynell, A. A., Balland, E., Lacombe, A., Mazur, D., Carmeliet, P. et al. (2013). Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 17, 607-617. https://doi.org/10.1016/j.cmet.2013.03.004Google ScholarLaursen, W. J., Mastrotto, M., Pesta, D., Funk, O. H., Goodman, J. B., Merriman, D. K., Ingolia, N., Shulman, G. I., Bagriantsev, S. N. and Gracheva, E. O. (2015). Neuronal UCP1 expression suggests a mechanism for local thermogenesis during hibernation. Proc. Natl. Acad. Sci. USA 112, 1607-1612. https://doi.org/10.1073/pnas.1421419112Google ScholarLewis, J. E. and Ebling, F. J. (2017). Tanycytes as regulators of seasonal cycles in neuroendocrine function. Front. Neurol. 8, 79. https://doi.org/10.3389/fneur.2017.00079Google ScholarLyman, C. P. and O'Brien, R. C. (1961). Circulatory changes in the 13-lined ground squirrel during the hibernating cycle. Tech. Rep. Arct. Aeromed. Lab. US 60, 1-18. https://doi.org/10.21236/AD0261757Google ScholarLyman, C. P. and O'Brien, R. C. (1963). Autonomic control of circulation during the hibernating cycle in ground squirrels. J. Physiol. 168, 477-499. https://doi.org/10.1113/jphysiol.1963.sp007204Google ScholarMann, F. C. (1916). The ductless glands and hibernation. Am. J. Physiol. 41, 173-188. https://doi.org/10.1152/ajplegacy.1916.41.2.173Google ScholarMargules, D. L., Goldman, B. and Finck, A. (1979). Hibernation: an opioid-dependent state? Brain Res. Bull. 4, 721-724. https://doi.org/10.1016/0361-9230(79)90003-0Google ScholarMatos-Cruz, V., Schneider, E. R., Mastrotto, M., Merriman, D. K., Bagriantsev, S. N. and Gracheva, E. O. (2017). Molecular prerequisites for diminished cold sensitivity in ground squirrels and hamsters. Cell Rep 21, 3329-3337. https://doi.org/10.1016/j.celrep.2017.11.083Google ScholarMcArthur, M. D. and Milsom, W. K. (1991). Changes in ventilation and respiratory sensitivity associated with hibernation in columbian (Spermophilus columbianus) and golden-mantled (Spermophilus lateralis) ground squirrels. Physiol. Zool. 64, 940-959. https://doi.org/10.1086/physzool.64.4.30157950Google ScholarMettler, F. A., Mettler, C. C. and Culler, E. (1935). Effects of total removal of the cerebral cortex. Arch. Neurol. Psychiatry 34, 1238-1249. https://doi.org/10.1001/archneurpsyc.1935.02250240107008Google ScholarMilsom, W. K., Harris, M. B. and Reid, S. G. (1997). Do descending influences alternate to produce episodic breathing? Respir. Physiol. 110, 307-317. https://doi.org/10.1016/S0034-5687(97)00096-0Google ScholarMilsom, W. K., Zimmer, M. B. and Harris, M. B. (1999). Regulation of cardiac rhythm in hibernating mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 124, 383-391. https://doi.org/10.1016/S1095-6433(99)00130-0Google ScholarMilsom, W. K., Zimmer, M. B. and Harris, M. B. (2001). Vagal control of cardiorespiratory function in hibernation. Exp. Physiol. 86, 791-796. https://doi.org/10.1111/j.1469-445X.2001.tb00046.xGoogle ScholarMin, S., Chang, R. B., Prescott, S. L., Beeler, B., Joshi, N. R., Strochlic, D. E. and Liberles, S. D. (2019). Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep 29, 2192-2201.e3. https://doi.org/10.1016/j.celrep.2019.10.040Google ScholarMohr, S. M., Bagriantsev, S. N. and Gracheva, E. O. (2020). Cellular, molecular, and physiological adaptations to hibernation: the solution to environmental challenges. Annu. Rev. Cell Dev. Biol. 36, 315-338. https://doi.org/10.1146/annurev-cellbio-012820-095945Google ScholarMorrison, S. F. and Nakamura, K. (2011). Central neural pathways for thermoregulation. Front. Biosci. 16, 74-104. https://doi.org/10.2741/3677Google ScholarMulawa, E. A., Kirkwood, J. S., Wolfe, L. M., Wojda, S. J., Prenni, J. E., Florant, G. L. and Donahue, S. W. (2018). Seasonal changes in endocannabinoid concentrations between active and hibernating marmots (Marmota flaviventris). J. Biol. Rhythms 33, 388-401. https://doi.org/10.1177/0748730418777660Google ScholarNilaweera, K., Herwig, A., Bolborea, M., Campbell, G., Mayer, C. D., Morgan, P. J., Ebling, F. J. and Barrett, P. (2011). Photoperiodic regulation of glycogen metabolism, glycolysis, and glutamine synthesis in tanycytes of the Siberian hamster suggests novel roles of tanycytes in hypothalamic function. Glia 59, 1695-1705. https://doi.org/10.1002/glia.21216Google ScholarPassmore, J. C., Pfeiffer, E. W. and Templeton, J. R. (1975). Urea excretion in the hibernating Columbian ground squirrel (Spermophilus columbianus). J. Exp. Zool. 192, 83-86. https://doi.org/10.1002/jez.1401920110Google ScholarPicardo, M. A., Merel, J., Katlowitz, K. A., Vallentin, D., Okobi, D. E., Benezra, S. E., Clary, R. C., Pnevmatikakis, E. A., Paninski, L. and Long, M. A. (2016). Population-level representation of a temporal sequence underlying song production in the Zebra finch. Neuron 90, 866-876. https://doi.org/10.1016/j.neuron.2016.03.038Google ScholarProvince, H. S., Xiao, C., Mogul, A. S., Sahoo, A., Jacobson, K. A., Piñol, R. A., Gavrilova, O. and Reitman, M. L. (2020). Activation of neuronal adenosine A1 receptors causes hypothermia through central and peripheral mechanisms. PLoS ONE 15, e0243986. https://doi.org/10.1371/journal.pone.0243986Google ScholarRasmussen, A. T. (1916). Theories of hibernation. Am. Nat. 50, 609-625. https://doi.org/10.1086/279571Google ScholarSallmen, T., Beckman, A. L., Stanton, T. L., Eriksson, K. S., Tarhanen, J., Tuomisto, L. and Panula, P. (1999). Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J. Neurosci. 19, 1824-1835. https://doi.org/10.1523/JNEUROSCI.19-05-01824.1999Google ScholarSaper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. and Scammell, T. E. (2010). Sleep state switching. Neuron 68, 1023-1042. https://doi.org/10.1016/j.neuron.2010.11.032Google ScholarSatinoff, E. (1964). Behavioral thermoregulation in response to local cooling of the rat brain. Am. J. Physiol. 206, 1389-1394. https://doi.org/10.1152/ajplegacy.1964.206.6.1389Google ScholarSchwartz, C., Hampton, M. and Andrews, M. T. (2013). Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS ONE 8, e58427. https://doi.org/10.1371/journal.pone.0058427Google ScholarSchwartz, C., Hampton, M. and Andrews, M. T. (2015). Hypothalamic gene expression underlying pre-hibernation satiety. Genes Brain Behav. 14, 310-318. https://doi.org/10.1111/gbb.12199Google ScholarScribner, J. L., Vance, E. A., Protter, D. S. W., Sheeran, W. M., Saslow, E., Cameron, R. T., Klein, E. M., Jimenez, J. C., Kheirbek, M. A. and Donaldson, Z. R. (2020). A neuronal signature for monogamous reunion. Proc. Natl. Acad. Sci. USA 117, 11076-11084. https://doi.org/10.1073/pnas.1917287117Google ScholarSekizawa, S., Bechtold, A. G., Tham, R. C. and Bonham, A. C. (2009). A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J. Neurosci. 29, 11807-11816. https://doi.org/10.1523/JNEUROSCI.2617-09.2009Google ScholarSekizawa, S., Horwitz, B. A., Horowitz, J. M. and Chen, C.-Y. (2013). Protection of signal processing at low temperature in baroreceptive neurons in the nucleus tractus solitarius of Syrian hamsters, a hibernating species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R1153-R1162. https://doi.org/10.1152/ajpregu.00165.2013Google ScholarSilvani, A., Cerri, M., Zoccoli, G. and Swoap, S. J. (2018). Is adenosine action common ground for NREM sleep, torpor, and other hypometabolic states? Physiology 33, 182-196. https://doi.org/10.1152/physiol.00007.2018Google ScholarSingh Alvarado, J., Goffinet, J., Michael, V., Liberti, W., III, Hatfield, J., Gardner, T., Pearson, J. and Mooney, R. (2021). Neural dynamics underlying birdsong practice and performance. Nature 599, 635-639. https://doi.org/10.1038/s41586-021-04004-1Google ScholarStrumwasser, F. (1958). Regulatory mechanisms, brain activity and behavior during deep hibernation in the squirrel, Citellus beecheyi. Am. J. Physiol. 196, 23-30. https://doi.org/10.1152/ajplegacy.1958.196.1.23Google ScholarTakahashi, T. M., Sunagawa, G. A., Soya, S., Abe, M., Sakurai, K., Ishikawa, K., Yanagisawa, M., Hama, H., Hasegawa, E., Miyawaki, A., et al. (2020). A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583, 109-114.Google ScholarTamura, Y., Shintani, M., Nakamura, A., Monden, M. and Shiomi, H. (2005). Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 1045, 88-96. https://doi.org/10.1016/j.brainres.2005.03.029Google ScholarTan, C. L. and Knight, Z. A. (2018). Regulation of body temperature by the nervous system. Neuron 98, 31-48. https://doi.org/10.1016/j.neuron.2018.02.022Google ScholarTorke, K. G. and Twente, J. W. (1977). Behavior of Spermophilus lateralis between periods of hibernation. J. Mammal. 58, 385-390. https://doi.org/10.2307/1379337Google ScholarTupone, D., Madden, C. J. and Morrison, S. F. (2013). Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J. Neurosci. 33, 14512-14525. https://doi.org/10.1523/JNEUROSCI.1980-13.2013Google ScholarTupone, D., Cano, G. and Morrison, S. F. (2017). Thermoregulatory inversion: a novel thermoregulatory paradigm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R779-R786. https://doi.org/10.1152/ajpregu.00022.2017Google ScholarWalker, J. M., Glotzbach, S. F., Berger, R. J. and Heller, H. C. (1977). Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am. J. Physiol. 233, R213-R221. https://doi.org/10.1152/ajpregu.1977.233.5.R213Google ScholarYackle, K., Schwarz, L. A., Kam, K., Sorokin, J. M., Huguenard, J. R., Feldman, J. L., Luo, L. and Krasnow, M. A. (2017). Breathing control center neurons that promote arousal in mice. Science 355, 1411-1415. https://doi.org/10.1126/science.aai7984Google ScholarZatzman, M. L. and South, F. E. (1981). Circannual renal function and plasma electrolytes of the marmot. Am. J. Physiol. 241, R87-R91. https://doi.org/10.1152/ajpregu.1981.241.1.R87Google ScholarZeng, W.-Z., Marshall, K. L., Min, S., Daou, I., Chapleau, M. W., Abboud, F. M., Liberles, S. D. and Patapoutian, A. (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362, 464-467. https://doi.org/10.1126/science.aau6324Google ScholarZimmerman, C. A., Lin, Y.-C., Leib, D. E., Guo, L., Huey, E. L., Daly, G. E., Chen, Y. and Knight, Z. A. (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680-684. https://doi.org/10.1038/nature18950Google ScholarZimmerman, C. A., Leib, D. E. and Knight, Z. A. (2017). Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18, 459-469. https://doi.org/10.1038/nrn.2017.71Google ScholarZimny, M. L., Franco, E. E., St Onge, M. and Pearson, J. (1984). Ultrastructure of juxtaglomerular cells correlated with biochemical parameters in a hibernator. Comp. Biochem. Physiol. A Comp. Physiol. 78, 229-235. https://doi.org/10.1016/0300-9629(84)90137-3
Close window by clicking in grey area >